- Department of Neurosurgery, Junshin Hospital, Kakogawa, Japan.
- Department of Neurosurgery, Kobe University Graduate School of Medicine, Kobe, Japan.
Correspondence Address:
Satoshi Inoue, Department of Neurosurgery, Junshin Hospital, Kakogawa, Japan.
DOI:10.25259/SNI_987_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Satoshi Inoue1, Atsushi Fujita2, Eiji Kurihara1, Takashi Sasayama2. Mechanical thrombectomy for acute paradoxical cerebral embolism due to pulmonary arteriovenous malformation: A case report and review of literature. 13-Jan-2023;14:13
How to cite this URL: Satoshi Inoue1, Atsushi Fujita2, Eiji Kurihara1, Takashi Sasayama2. Mechanical thrombectomy for acute paradoxical cerebral embolism due to pulmonary arteriovenous malformation: A case report and review of literature. 13-Jan-2023;14:13. Available from: https://surgicalneurologyint.com/surgicalint-articles/12100/
Abstract
Background: We report a case of acute occlusion of the middle cerebral artery (MCA) due to pulmonary arteriovenous malformation (PAVM), for which mechanical thrombectomy was performed and a good outcome was achieved.
Case Description: A 59-year-old woman presented with severe right hemiplegia and dysarthria, and a National Institutes of Health Stroke Scale (NIHSS) score of 10. Magnetic resonance imaging (MRI) diffusion-weighted imaging revealed high-signal areas in the left lenticular nucleus to the corona radiata, and right temporal lobe. Magnetic resonance angiography revealed the left MCA occlusion. Chest plain computed tomography (CT) revealed PAVM in the lower lobe of the left lung. Transvenous thrombolytic therapy was started 2 h after onset, and mechanical cerebral thrombectomy was performed with an aspiration catheter and stent retriever. A red thrombus was retrieved in one pass and complete recanalization was achieved. After treatment, her symptoms improved markedly, but a symptomatic intracerebral hematoma was observed in the contralateral right temporal lobe. The patient underwent embolization for PAVM on day 24 and was transferred for rehabilitation (NIHSS score = 2).
Conclusion: Although rare, PAVM is a treatable cause of stroke in relatively young adults; thus, it is important to keep in mind the possibility of its presence.
Keywords: Acute ischemic stroke, Hereditary hemorrhagic telangiectasia, Paradoxical cerebral embolization, Recombinant tissue plasminogen activator, Stent retriever
INTRODUCTION
Pulmonary arteriovenous malformation (PAVM), or pulmonary arteriovenous fistula, is a rare vascular anomaly in which dilated abnormal vessels directly connect the pulmonary and systemic circulation without capillaries.[
Although the efficacy of acute recanalization therapy for ischemic stroke is well established, there are few reports for cerebral embolism associated with PAVM.[
CASE PRESENTATION
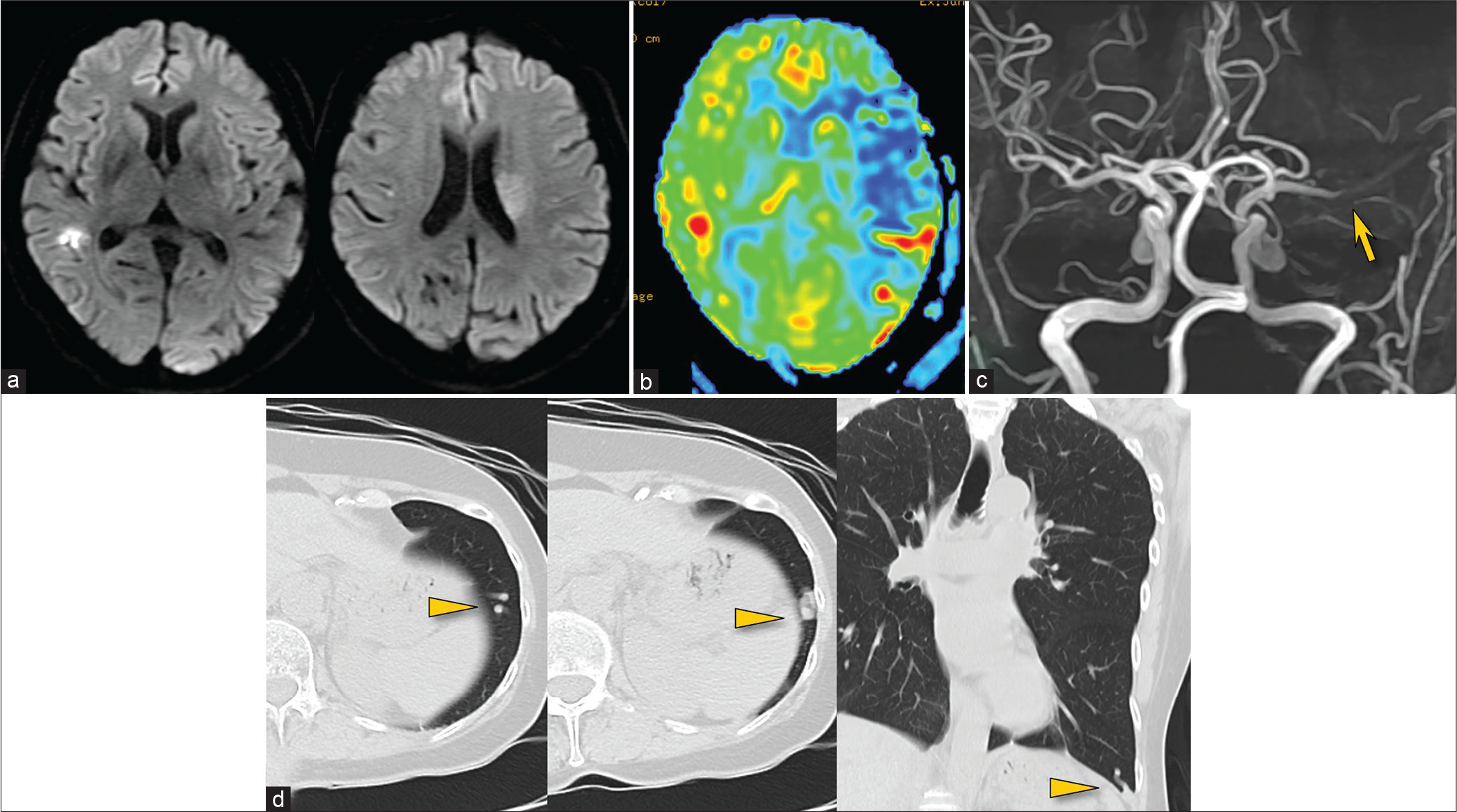
A 59-year-old woman with no medical or family history was brought to the emergency room at 7:50 AM with the right hemiplegia that occurred while she was taking a bath. At the time of initial examination, she had Glasgow Coma Scale 14 (E4V4M6), amenable but slow response, and severe right hemiplegia (Manual Muscle Test 1/5) with marked dysarthria. She was diagnosed with a National Institutes of Health Stroke Scale (NIHSS) score of 10. MRI diffusion-weighted imaging (DWI) showed a vague high-signal area in the left lenticular nucleus and corona radiata, and a small clear lesion in the temporal lobe, with a DWI-Alberta Stroke Program Early Computed Tomography (CT) Score of 9/11 [
Figure 1:
MR diffusion-weighted imaging showing a vague left ischemic lesion and a small right lesion (a), perfusion imaging showing diffuse left hypoperfusion (b), and MR angiography showing left middle cerebral artery occlusion (arrow) (c). Chest CT showing a pulmonary arteriovenous malformation in the left lung (arrowheads) (d).
MT for left MCA occlusion
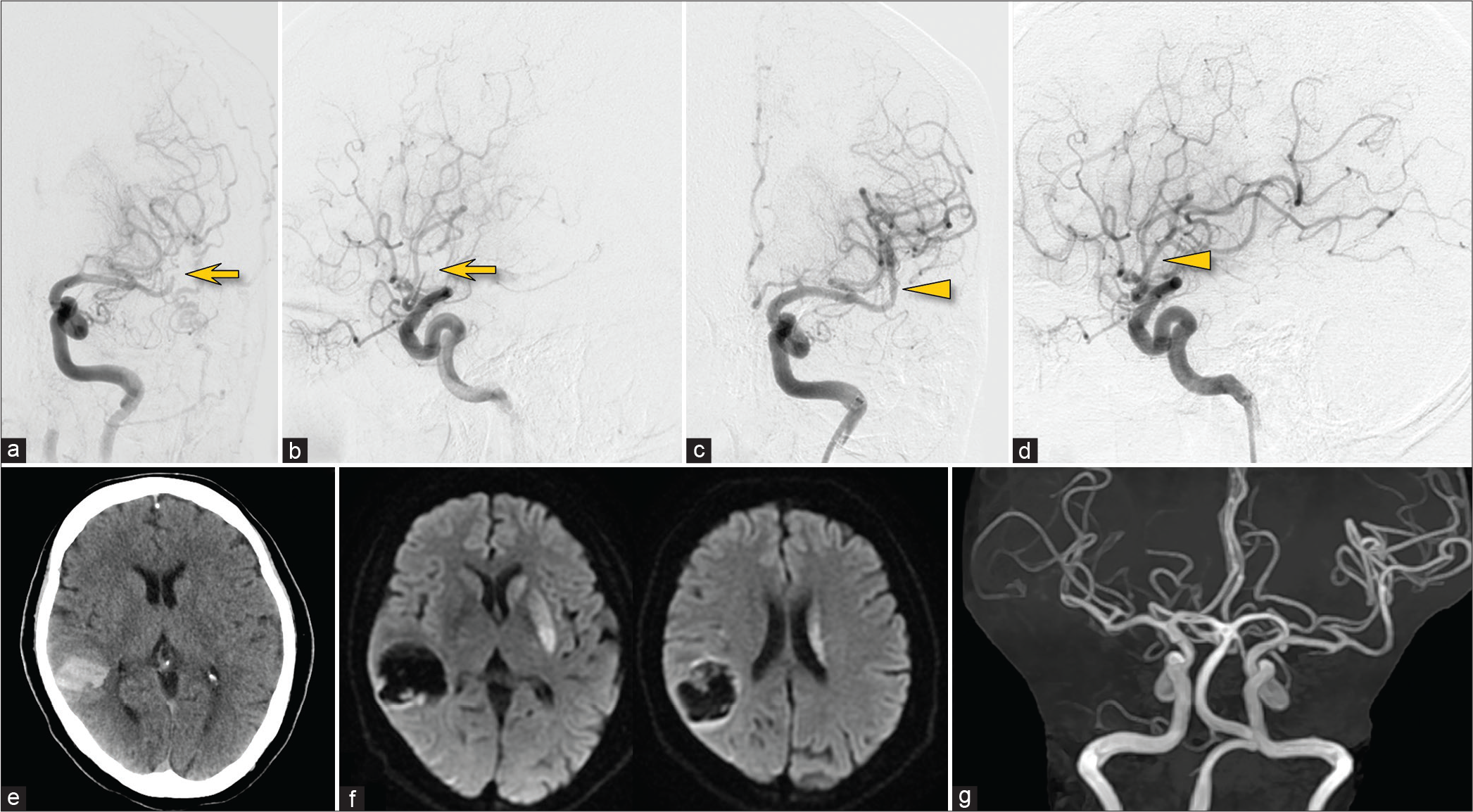
The right femoral artery was punctured under local anesthesia (10:22 AM), and a 9F Optimo (Tokai Medical Products, Aichi, Japan) was inserted into the left internal carotid artery. Heparin-added saline was used for irrigation of each catheter, but heparin bolus administration was avoided because the patient was receiving rtPA. Angiography showed occlusion of the M2 segment of the left MCA [
Figure 2:
Left internal carotid arteriography before (a and b) and after treatment (c and d). The occlusion of the left middle cerebral artery (arrows) was successfully recanalized (arrowheads). Post-treatment CT showing a contralateral intracerebral hematoma (e). Follow-up MR imaging (f) and MR angiography (g) showing no evidence of recurrent stroke.
Immediately after treatment, the patient became conscious, and her severe right hemiplegia improved markedly. Head CT showed an intracerebral hematoma in the contralateral right temporal lobe, but no new abnormal findings were found on the treated side [
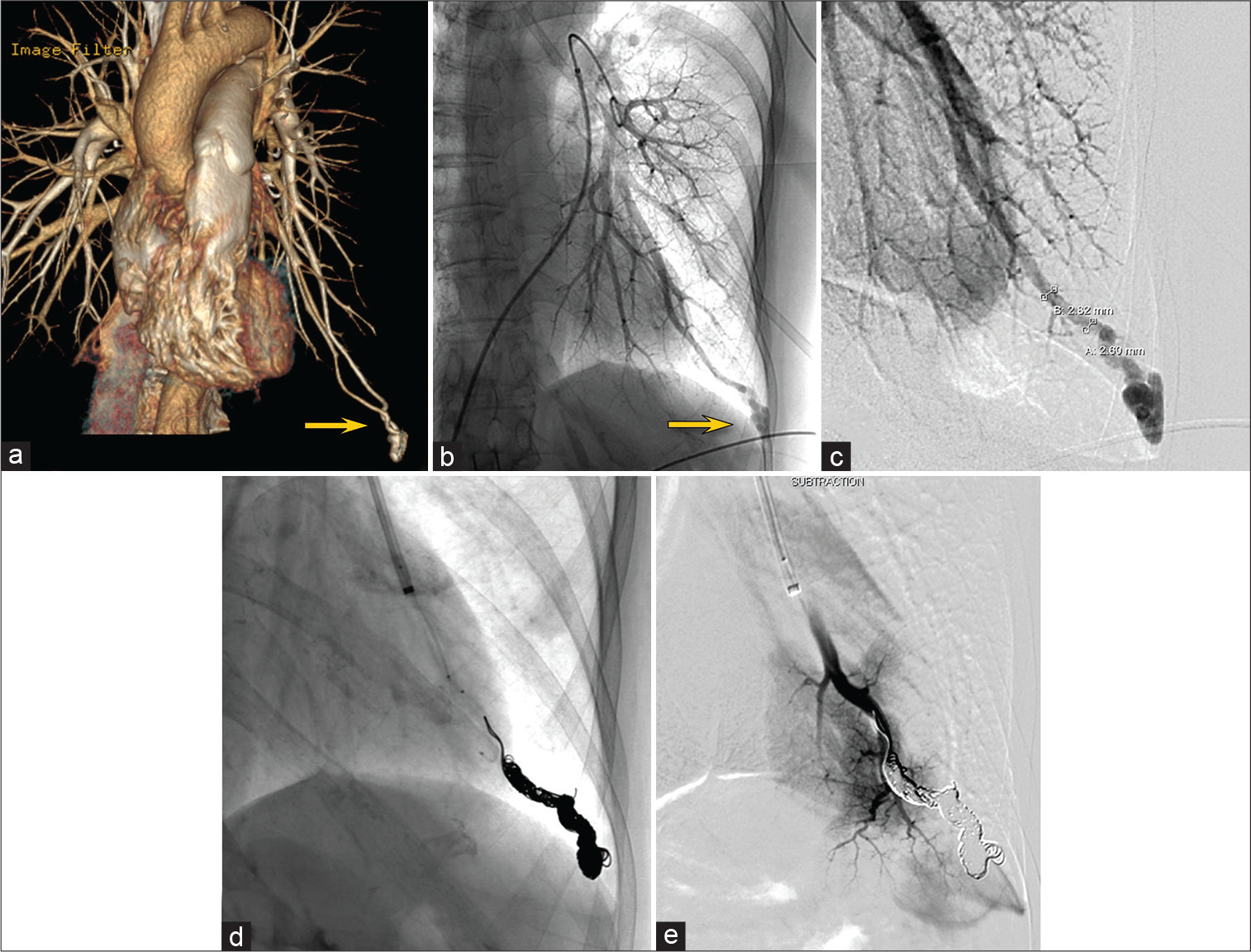
Coil embolization of the left pulmonary PAVM
Under local anesthesia and systemic heparinization, an 8F Optimo was inserted from the right femoral vein into a branch of the left pulmonary artery, the feeder of the PAVM. The PAVM was a single lesion, simple type with a single feeder (2.6–2.8 mm in diameter), and a single drainer [
The patient’s post-treatment course was uneventful. Her left homonymous hemianopsia and left hemispatial neglect remained but tended to improve. She was transferred for rehabilitation on day 46 without stroke recurrence (NIHSS 2, modified Rankin Scale 2).
DISCUSSION
Literature review of acute recanalization therapy for stroke with PAVM
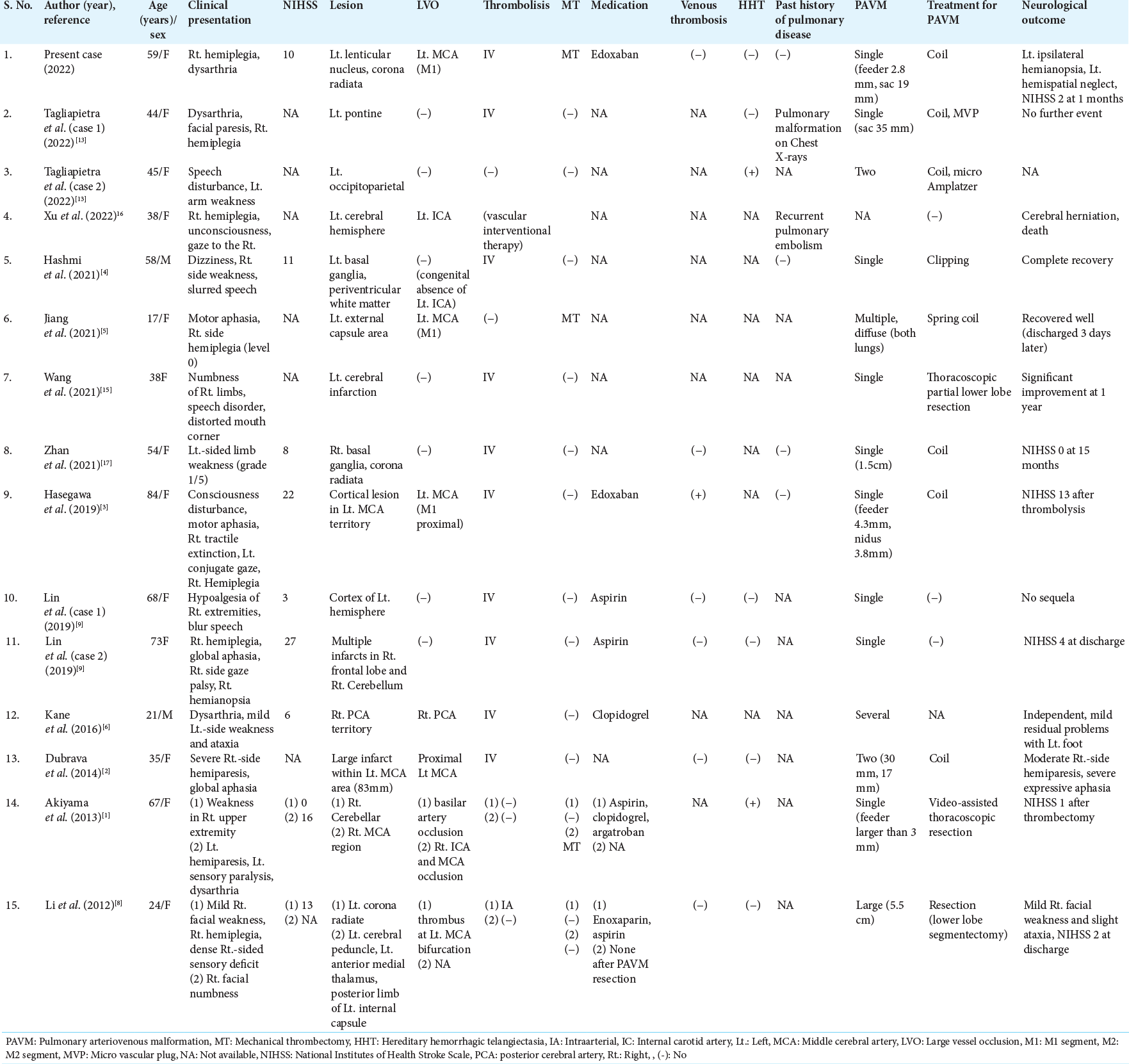
The prevalence of PAVM in acute ischemic stroke is rare, ranging from 0.02% to 0.5%.[
Evidence for recanalization therapy for stroke with PAVM
At present, there are no practical guidelines for acute recanalization therapy for PAVM-related paradoxical cerebral embolism. According to the British Thoracic Society Clinical Statement, there is no evidence for the safety and efficacy of thrombolytic therapy.[
There is also no evidence for optimal antithrombotic management after acute recanalization therapy for cerebral embolism due to PAVM. In the present review, antiplatelet agents were administered in five patients and anticoagulants in two patients. However, two patients had early recurrence of ischemic stroke despite receiving antiplatelet therapy.[
Diagnosis of PAVM in the acute phase of stroke
Because PAVM is rare and cerebral infarction is often the initial presentation,[
MT for stroke with PAVM
In cases of cerebral large vessel occlusion due to PAVM, MT may be less risky than thrombolysis in terms of hemorrhagic complications, especially when HHT is present.[
Treatment of PAVM
Endovascular embolization is the first-line treatment for PAVM.[
CONCLUSION
We report a case of acute recanalization therapy with MT for a cerebral embolism due to PAVM. Although rare, PAVM is a treatable condition that can cause ischemic stroke in young adults; thus, it is important to keep in mind the possibility of its presence.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors thank Dr. Hiroaki Nakamura and Dr. Takeshi Hatta for the appropriate management and treatment of PAVM.
References
1. Akiyama S, Hanada S, Uruga H, Takaya H, Miyamoto A, Morokawa N. Hereditary hemorrhagic telangiectasia with pulmonary arteriovenous malformation and embolic strokes treated successfully with video-assisted thoracoscopic resection. Intern Med. 2013. 52: 1091-4
2. Dubrava J, Vulev I, Richter D. Isolated pulmonary arteriovenous fistulas with massive right-to-left shunt as a rare cause of cryptogenic stroke in a young woman. Isr Med Assoc J. 2014. 16: 320-1
3. Hasegawa I, Abe T, Mino T, Okamoto K, Takeda A, Itoh Y. Paradoxical brain embolism caused by isolated pulmonary arteriovenous fistula successfully treated with recombinant tissue plasminogen activator. J Stroke Cerebrovasc Dis. 2019. 28: e100-1
4. Hashmi AT, Batool A, Khalid MO, Raheja H, Sadiq A, Hollander G. Multiple strokes due to pulmonary arteriovenous malformation. Radiol Case Rep. 2021. 16: 2362-5
5. Jiang X, He L, Shen B, Ma H, Zhang B. Pulmonary multifocal arteriovenous malformations lead to ischemic stroke in young adults: A case report and literature review. Ann Palliat Med. 2021. 10: 12034-8
6. Kane I, Ford AP, Lawton K, Poitelea M, Gainsborough N. Ischaemic stroke in a 21-year-old with hereditary haemorrhagic telangiectasia. Pract Neurol. 2016. 16: 381-4
7. Kramdhari H, Valakkada J, Ayyappan A. Diagnosis and endovascular management of pulmonary arteriovenous malformations. Br J Radiol. 2021. 94: 20200695
8. Li Y, Margraf J, Kluck B, Jenny D, Castaldo J. Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: A case report and literature review. Neurologist. 2012. 18: 44-8
9. Lin G, Jiang P, Lou M. Thrombolysis in ischemic stroke patients with isolate pulmonary arteriovenous malformations. J Stroke Cerebrovasc Dis. 2019. 28: e68-70
10. Müller-Hülsbeck S, Marques L, Maleux G, Osuga K, Pelage JP, Wohlgemuth WA. CIRSE standards of practice on diagnosis and treatment of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. 2020. 43: 353-61
11. Shimohira M, Kawai T, Hashizume T, Muto M, Kitase M, Shibamoto Y. Usefulness of hydrogel-coated coils in embolization of pulmonary arteriovenous malformations. Cardiovasc Intervent Radiol. 2018. 41: 848-55
12. Shovlin CL, Condliffe R, Donaldson JW, Kiely DG, Wort SJ. British thoracic society clinical statement on pulmonary arteriovenous malformations. Thorax. 2017. 72: 1154-63
13. Tagliapietra M, Turri G, Bortolotti F, Mansueto G, Monaco S. Ischemic stroke due to sporadic and genetic pulmonary arteriovenous malformations: Case report. Brain Circ. 2022. 8: 57-60
14. Topiwala KK, Patel SD, Saver JL, Streib CD, Shovlin CL. Ischemic stroke and pulmonary arteriovenous malformations: A review. Neurology. 2022. 98: 188-98
15. Wang J, Zhang J, Wang A, Wang C. Pulmonary arteriovenous fistula diagnosed by transthoracic contrast echocardiography: A case report. J Clin Ultrasound. 2021. 49: 298-300
16. Xu S, Hong M, Zhou Z. Images of the month 1: Ischaemic stroke due to pulmonary arteriovenous fistula. Clin Med (Lond). 2022. 22: 278-9
17. Zhan J, Dong C, Li M, Zhan L, Chen H, Lu L. Cryptogenic stroke caused by pulmonary arterial venous malformation with massive right-to-left shunt: A case report. Neurol Ther. 2021. 10: 1135-42