- Department of Neurosurgery, Shizuoka City Shimizu Hospital, Shizuoka, Japan.
Correspondence Address:
Mizuto Sato, Department of Neurosurgery, Shizuoka City Shimizu Hospital, Shizuoka, Japan.
DOI:10.25259/SNI_73_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mizuto Sato, Yoichi Mochizuki, Masahito Fukuchi, Koji Fujii. Middle meningeal artery embolization before craniotomy for infected organizing chronic subdural hematoma: A case report and review of the literature. 06-May-2022;13:186
How to cite this URL: Mizuto Sato, Yoichi Mochizuki, Masahito Fukuchi, Koji Fujii. Middle meningeal artery embolization before craniotomy for infected organizing chronic subdural hematoma: A case report and review of the literature. 06-May-2022;13:186. Available from: https://surgicalneurologyint.com/surgicalint-articles/11585/
Abstract
Background: Organizing chronic subdural hematoma (OSDH) is intractable and its radical treatment remains controversial. Middle meningeal artery embolization has emerged as an adjunctive treatment to craniotomy for OSDH.
Case Description: The patient is an 86-year-old man. He had been taking warfarin for atrial fibrillation and was referred to the department for the treatment of bilateral chronic subdural hematoma (CSDH), which was found on head computed tomography after a fall. Bilateral burr hole drainages were performed, but his hematomas were organized, so the hematomas could not be drained sufficiently. The patient was discharged from the hospital without any neurological symptoms. Two months later, the patient presented with persistent fever and headache and had recurrent bilateral CSDHs. The hematoma on the right side was larger. Based on the initial intraoperative findings, OSDH was suspected, and craniotomy was performed on the right hematoma. Propionibacterium acnes were detected in the hematoma culture, and antimicrobial therapy was started postoperatively. Since the right hematoma recurred on the 7th postoperative day, bilateral middle meningeal artery (MMA) embolization with 20% n-butyl-2-cyanoacrylate was performed, followed by craniotomy for the left hematoma and drainage for the right recurrent hematoma. Antimicrobials were administered for 2 weeks after the last operations. Six months after the operations, both bilateral hematomas had almost disappeared.
Conclusion: Craniotomy is effective for the treatment of infected OSDH, and MMA embolization is useful to reduce the risk of bleeding complications in the perioperative period, and may also reduce the recurrence of CSDH.
Keywords: Chronic subdural hematoma, Middle meningeal artery embolization, Organizing subdural hematoma, Propionibacterium acnes
INTRODUCTION
Chronic subdural hematoma (CSDH) is believed to be caused by disruption of the dural border cell layer in the dura mater due to minor head trauma, angiogenesis, and septal formation from hemorrhage, and resulting repeated microbleeding.[
The morbidity of CSDH is believed to increase with patient age, and an increasing number of patients take antithrombotic drugs, such as warfarin, DOACs, and antiplatelet drugs.[
Since the first report of MMA embolization as a treatment for CSDH in 2000,[
CASE DESCRIPTION
The patient was an 86-year-old man with a history of atrial fibrillation; he had been taking warfarin. The patient was referred to the department because bilateral CSDHs were found on the computed tomography (CT) scan, which was performed as an examination of his unsteadiness. The head CT showed bilateral convex lenticular hematomas with relatively high density but no calcification [
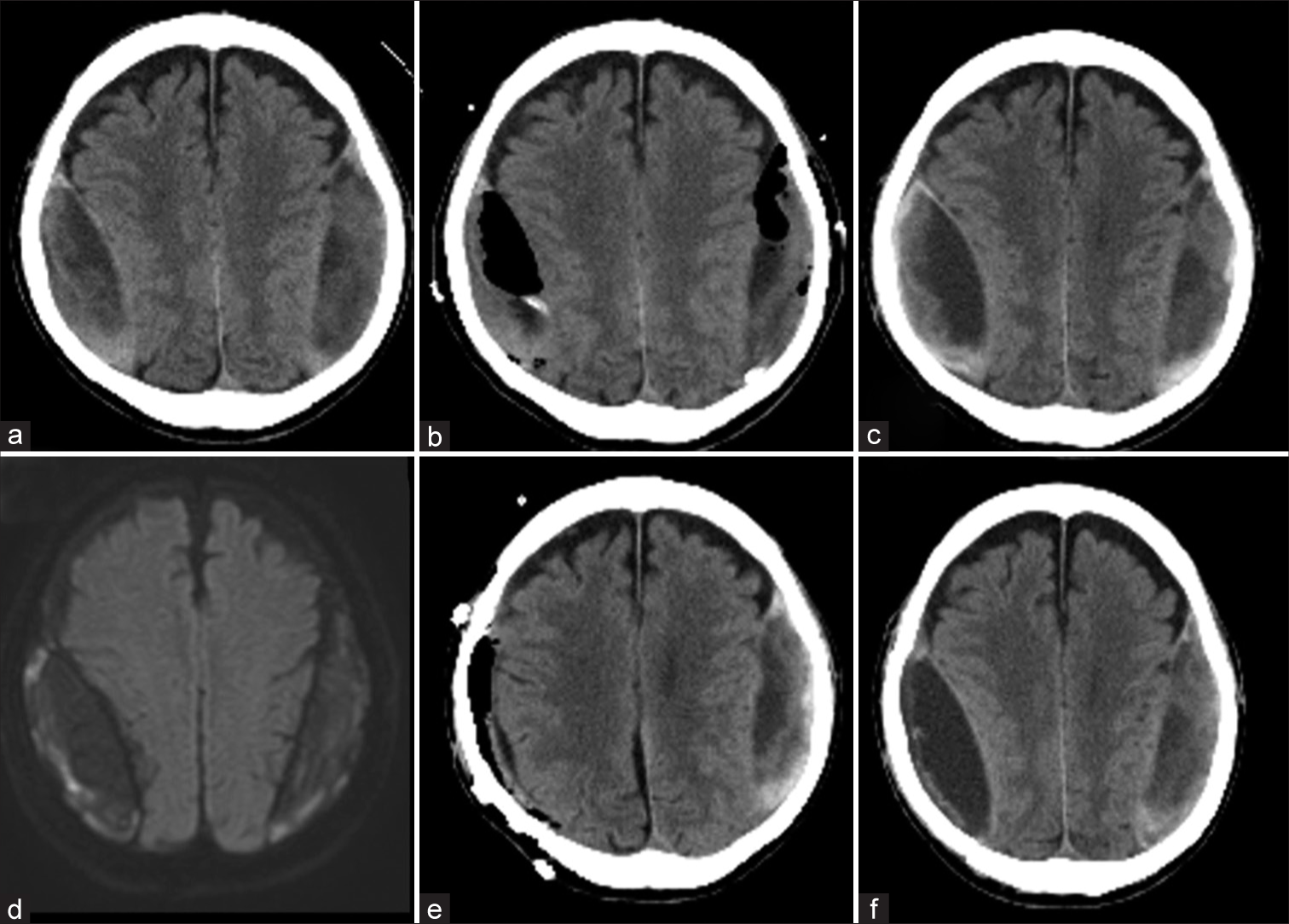
Figure 1:
Imaging history of the patient before middle meningeal artery (MMA) embolization: the patient had bilateral biconvex lenticular hematoma (a). Bilateral burr hole irrigation was performed (b). Two months after his discharge, the hematoma was recurred (c), and MRI showed high DWI image (d). After the craniotomy for the right hematoma (e), the right side hematoma recurred in 2 weeks (f).
Two months after discharge from the hospital, the patient presented with a complaint of slight fever and headache that persisted for several days. The blood test performed at that time showed a mild elevation of serum C-reactive protein at 4.73 mg/dL. Although the CSDH had not increased significantly since the last time [
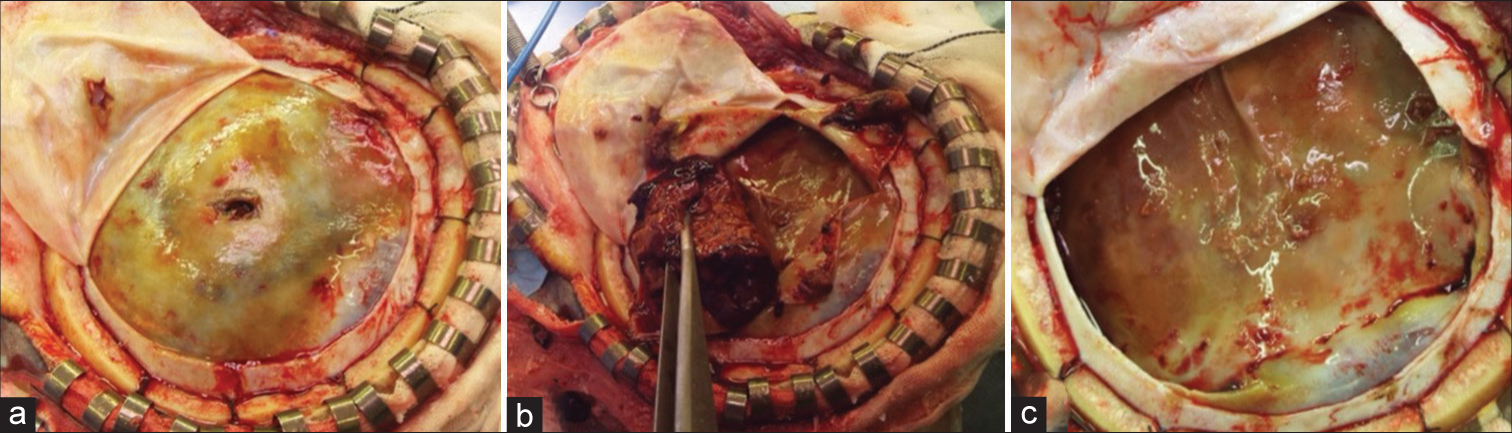
The intraoperative findings showed that the dura was incised and a yellow hematoma outer membrane was evident. The hematoma was hard and organizing and was removed completely. The entire hematoma inner membrane was exposed. There were several areas of microscopic bleeding from the border between the outer and inner membrane, and the entire area was completely cauterized to hemostasis. Although an infectious hematoma was suspected, the inner membrane was preserved due to the fear of spreading the bacteria to the brain surface, and a risk of brain parenchymal damage. The hematoma was examined for culture, and Propionibacterium acnes were detected, leading to the diagnosis of infectious OSDH. The patient was treated with ampicillin/sulbactam from the postoperative period. Two weeks after surgery, a CT scan showed recurrence of the right hematoma [
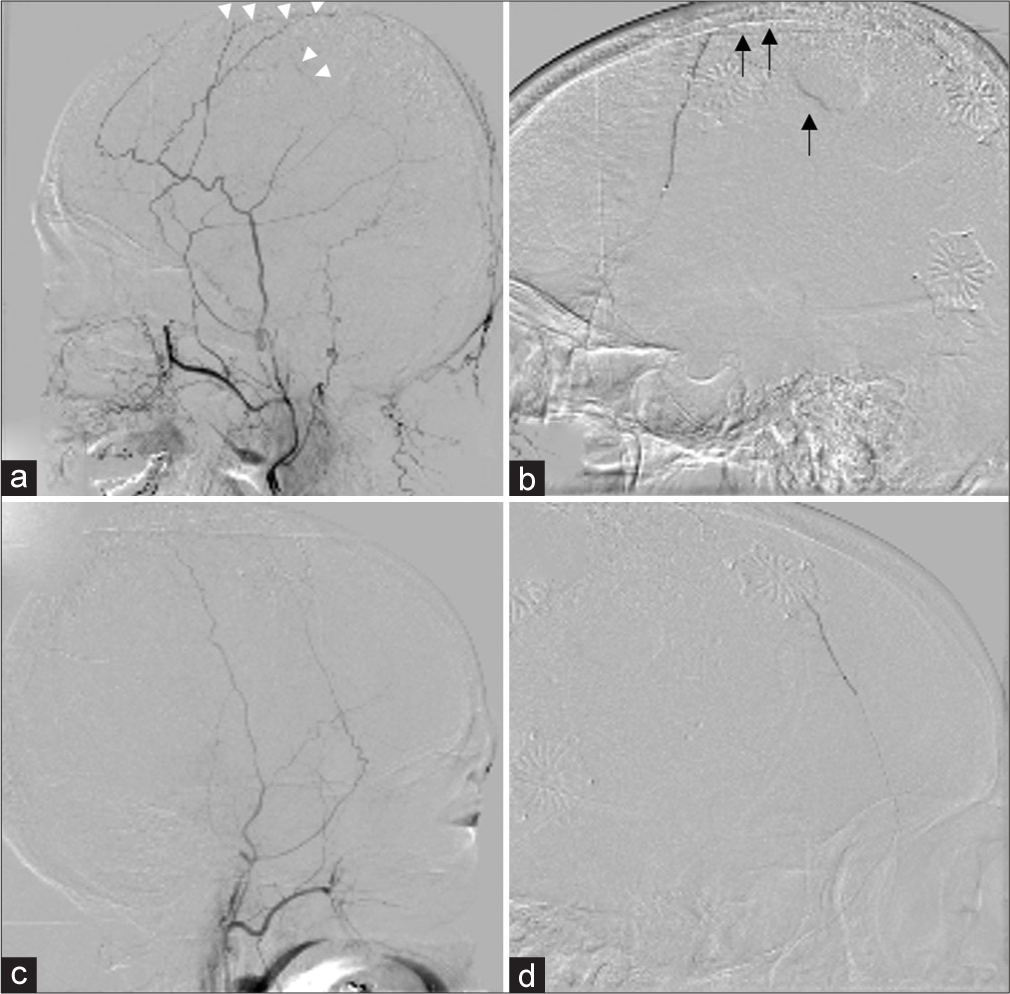
The MMA embolization revealed that the left external carotid artery imaging showed the vascular foci feeding the hematoma [
Figure 2:
Middle meningeal artery (MMA) embolization was performed. The left external carotid artery imaging showed the vascular foci feeding the hematoma mainly in the parietal region (a: white arrows), and embolization with 20% n-butyl-2-cyanoacrylate (NBCA) was performed (b: black arrows). The right external carotid artery imaging showed no abnormal vessels reaching the hematoma due to the postoperative craniotomy (c), but the remaining MMA branch was embolized with 20% NBCA (d).
The day after embolization, a craniotomy was performed for the left hematoma and drainage for the right hematoma under general anesthesia. The findings of the left craniotomy were the same as those of the right craniotomy: yellow outer membrane [
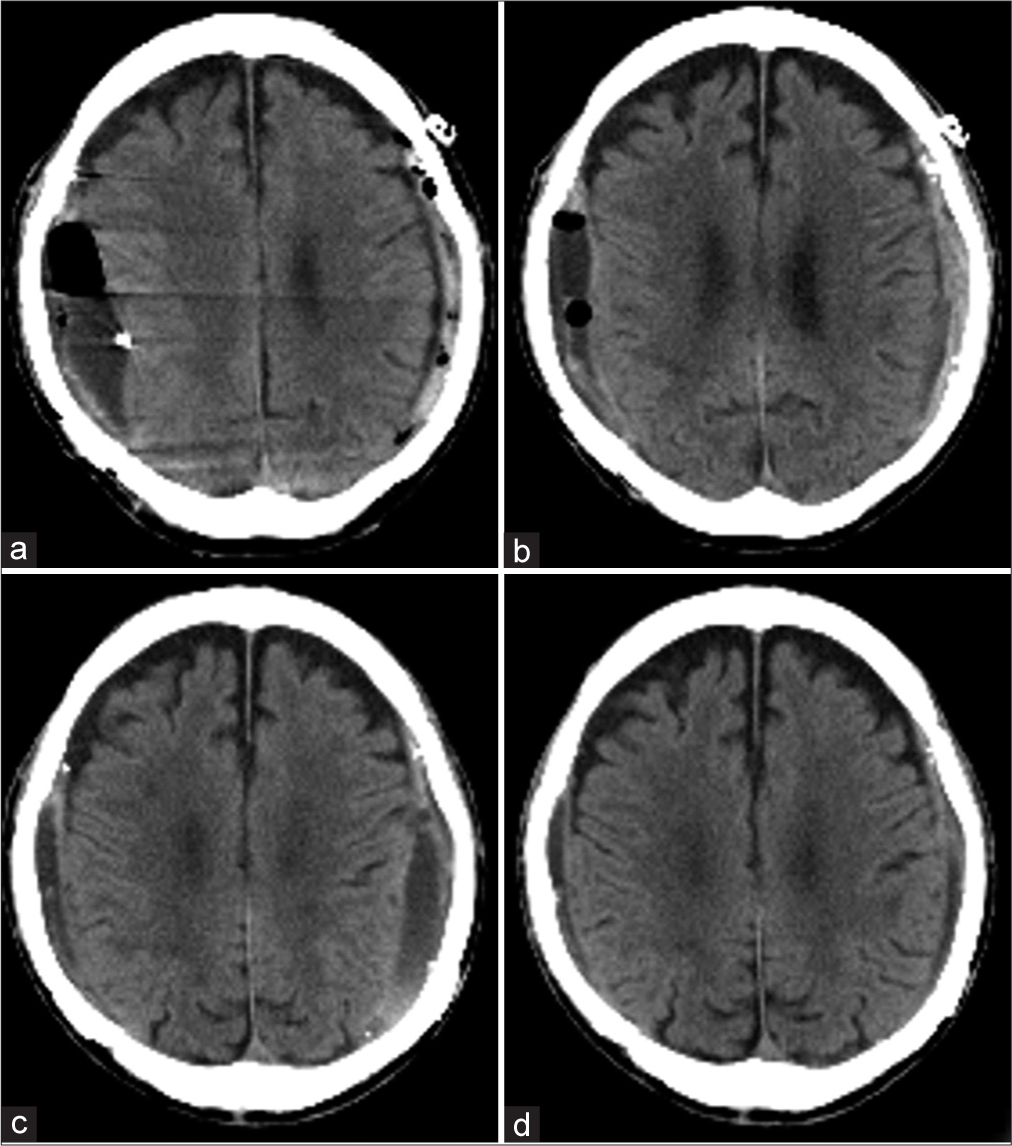
Six months after the surgery, the hematoma had almost disappeared on CT imaging [
DISCUSSION
CSDH is believed to be the result of the disruption of the dural border cell layer in the dura mater due to minor trauma, which leads to the formation of new blood vessels and septum within the hematoma.[
OSDH occurs in 0.5–2.0% of all CSDHs and is considered hard to treat radically.[
Preoperative diagnosis of an OSDH is difficult and cannot be made on plain CT unless it is accompanied by calcification.[
Morphologic changes in CSDH may suggest hematoma infection, especially in elderly patients, even when the results of blood tests and DWI findings are not typical for abscesses. Not only history, neurological examination, and blood tests but also radiological features, especially CT imaging, have been reported to show biconvex lenticular hematomas.[
As for cerebral angiographic findings, in selective imaging of the MMA, an abnormal network of punctate, mottled, and band-like vessels along the entire dilated and branched MMA can be seen mainly after the middle arterial phase. These are capillary-like vessels that exist in the hematoma outer membrane and it is thought that arterial pressure from the MMA disrupts these vessels, causing bleeding near the outer membrane. The diameter of these small arteries is reported to be as small as 50 µm.[
Although craniotomy may be considered preferable for the radical treatment of OSDHs, acute subdural hematoma complications after craniotomy have been reported in 6–12% of patients.[
MMA embolization for CSDH was first reported in 2000,[
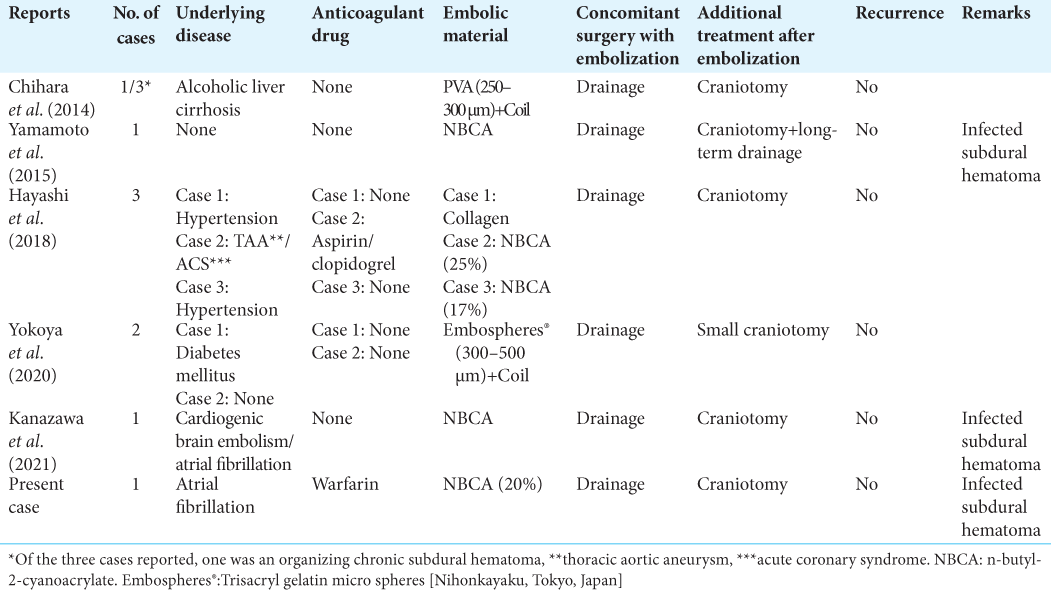
Anatomically, anastomosis of the MMA and ophthalmic artery through the recurrent meningeal artery is commonly recognized as dangerous anastomosis and requires attention during embolization. MMA embolization has been used for OSDH as well. As embolization material, PVA + coil, trisacryl gelatin microspheres (Embosphere®, Nihonkayaku, Tokyo, Japan), and NBCA have been used, and all of them have been reported to have some success [
In this case, the patient was taking warfarin for atrial fibrillation, and there was concern about postoperative bleeding during craniotomy. The biconvex lenticular hematomas and the high diffusion-weighted imaging signal suggested the possibility of infectious CSDH. It was thought that craniotomy was necessary to reduce the number of bacteria. Although there is no clear consensus on the radical treatment of infectious CSDH, early craniotomy is recommended for bacterial debulking.[
CONCLUSION
As a treatment for organic chronic subdural hematoma, MMA embolization may avoid bleeding complications during and after craniotomy, as well as prevent recurrence of chronic subdural hematoma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Enago (www.enago.jp ) for the English language review.
References
1. Akaishi T, Karibe H, Endo T, Ishii T. Organized chronic subdural hematoma: A condition presenting mixed-density hematoma without gradation density on brain CT. Case Rep Neurol. 2021. 13: 699-703
2. Balevi M. Organized chronic subdural hematomas treated by large craniotomy with extended membranectomy as the initial treatment. Asian J Neurosurg. 2017. 12: 598-604
3. Callovini GM, Bolognini A, Callovini G, Gammone V. Primary enlarged craniotomy in organized chronic subdural hematomas. Neurol Med Chir (Tokyo). 2014. 54: 349-56
4. Chen K, Wang K, Chen D, Niu H, Yang S, Wang Y. Surgical procedure in the treatment of organized chronic subdural hematoma: A single-center experience. J Neurol Surg A Cent Eur Neurosurg. 2021. 82: 241-7
5. Chihara H, Imamura H, Ogura T, Adachi H, Imai Y, Sakai N. Recurrence of a refractory chronic subdural hematoma after middle meningeal artery embolization that required craniotomy. NMC Case Rep J. 2014. 1: 1-5
6. Dabdoub CB, Adorno JO, Urbano J, Silveira EN, Orlandi BM. Review of the management of infected subdural hematoma. World Neurosurg. 2016. 87: 663.e1-8
7. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017. 14: 108
8. Fujioka M, Okuchi K, Miyamoto S, Sakaki T, Tsunoda S, Iwasaki S. Bilateral organized chronic subdural haematomas: High field magnetic resonance images and histological considerations. Acta Neurochir (Wien). 1994. 131: 265-9
9. Haldrup M, Ketharanathan B, Debrabant B, Schwartz OS, Mikkelsen R, Fugleholm K. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir (Wien). 2020. 162: 777-84
10. Hayashi S, Nishimoto Y, Nonakla M, Higuchi S, Hosoda K, Miki T. Craniotomy following middle meningeal artery embolization for organized chronic subdural hematoma: Three case reports. Neurosurg Emerg. 2018. 23: 138-4
11. Imaizumi S, Onuma T, Kameyama M, Naganuma H. Organized chronic subdural hematoma requiring craniotomy--five case reports. Neurol Med Chir (Tokyo). 2001. 41: 19-24
12. Kanazawa T, Karatsu K, Kuramae T, Ishihara M. Postoperative infected organised subdural haematoma that necessitated wide craniotomy. BMJ Case Rep. 2021. 14: e245134
13. Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000. 93: 686-8
14. Rodziewicz GS, Chuang WC. Endoscopic removal of organized chronic subdural hematoma. Surg Neurol. 1995. 43: 569-73
15. Takahashi S, Yazaki T, Nitori N, Kano T, Yoshida K, Kawase T. Neuroendoscope-assisted removal of an organized chronic subdural hematoma in a patient on bevacizumab therapy--case report. Neurol Med Chir (Tokyo). 2011. 51: 515-8
16. Tamai S, Watanabe T, Ichinose T, Murakami KI, Ueno M, Munemoto S. Morphological characteristics of infected subdural hematoma: Comparison with images of chronic subdural hematoma. Clin Neurol Neurosurg. 2020. 194: 105831
17. Tamura R, Sato M, Yoshida K, Toda M. History and current progress of chronic subdural hematoma. J Neurol Sci. 2021. 429: 118066
18. Tanaka T, Kaimori M. Histological study of vascular structure between the dura mater and the outer membrane in chronic subdural hematoma in an adult. No Shinkei Geka. 1999. 27: 431-6
19. Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: Systematic review and case series. World Neurosurg. 2019. 126: 228-36
20. Yokoya S, Nishii S, Takezawa H, Katsumori T, Takagi Y, Goto Y. Organized chronic subdural hematoma treated with middle meningeal artery embolization and small craniotomy: Two case reports. Asian J Neurosurg. 2020. 15: 421-4