- Department of Neurosurgery, Instituto Nacional de Ciencias Neurologicas, Lima, Peru
- Department of Neurosurgery, Hospital Guillermo Almenara Irigoyen, Lima, Peru.
Correspondence Address:
John Vargas-Urbina, Department of Neurosurgery, Instituto Nacional de Ciencias Neurologicas, Lima, Peru.
DOI:10.25259/SNI_28_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: John Vargas-Urbina1, John Alex Crisanto-Silva1, Carlos Vásquez-Perez1, Aarón Davila-Adrianzén1, Daniel Alcas-Seminario1, William Lines-Aguilar1, Rocio Mamani-Choquepata1, Giuseppe Panta-Rojas2. Multimodal management of giant solid hemangioblastomas in two patients with preoperative embolization. 26-Apr-2024;15:144
How to cite this URL: John Vargas-Urbina1, John Alex Crisanto-Silva1, Carlos Vásquez-Perez1, Aarón Davila-Adrianzén1, Daniel Alcas-Seminario1, William Lines-Aguilar1, Rocio Mamani-Choquepata1, Giuseppe Panta-Rojas2. Multimodal management of giant solid hemangioblastomas in two patients with preoperative embolization. 26-Apr-2024;15:144. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12873
Abstract
Background: Hemangioblastomas are benign vascular neoplasms, World Health Organization grade I, with the most frequent location in the cerebellum. Complete microsurgical resection can be a challenge due to excessive bleeding, which is why preoperative embolization takes importance.
Case Description: Two clinical cases are presented, a 25-year-old woman and a 75-year-old man, who presented with intracranial hypertension symptoms due to obstructive hydrocephalus; a ventriculoperitoneal shunt was placed in both cases; in addition, they presented with cerebellar signs. Both underwent embolization with ethylene vinyl alcohol copolymer, with blood flow reduction. After that, they underwent microsurgical resection within the 1st-week post embolization, obtaining, in both cases, gross total resection without hemodynamic complications, with clinical improvement and good surgical outcome. It is worth mentioning that surgical management is the gold standard that allows a suitable surgical approach, like in our patients, for which a lateral suboccipital craniotomy was performed.
Conclusion: Solid hemangioblastomas are less frequent than their cystic counterparts. The treatment is the surgical resection, which is a challenge and always has to be considered as an arteriovenous malformation in the surgical planning, including preoperative embolization to reduce perioperative morbidity and mortality and get good outcomes.
Keywords: Hemangioblastoma, Infratentorial neoplasms, Posterior cranial fossa, Surgical blood loss, Therapeutic embolization (source: MeSH-BIREME)
INTRODUCTION
Intracranial hemangioblastomas are benign vascular neoplasms, considered World Health Organization (WHO) grade I, developing mostly in the posterior fossa; the usual locations are the cerebellum (44–72%), spinal cord (13–44%), and brainstem (1–6%).[
The majority of hemangioblastomas are cystic or multicystic, reaching up to 70–75%.[
The complete microsurgical resection of this tumor can be challenging due to excessive bleeding from this very vascularized tumor,[
Preoperative embolization improves safety and effectiveness, allowing a cleaner and unobstructed surgical field due to bleeding, which results in a shorter surgery duration and less perioperative morbidities.[
CASE DESCRIPTION
In our hospital, we have between five to ten cystic hemangioblastoma cases per year, and it is rare to have cases of purely solid hemangioblastomas. Here, we present two cases that went to surgery in 2022, but at the follow-up in 2024, they show small recurrences despite images that show total resection in the early follow-up. It is worth mentioning that our patients do not have closer follow-up due to the distance from the specialized institutes.
Case 1
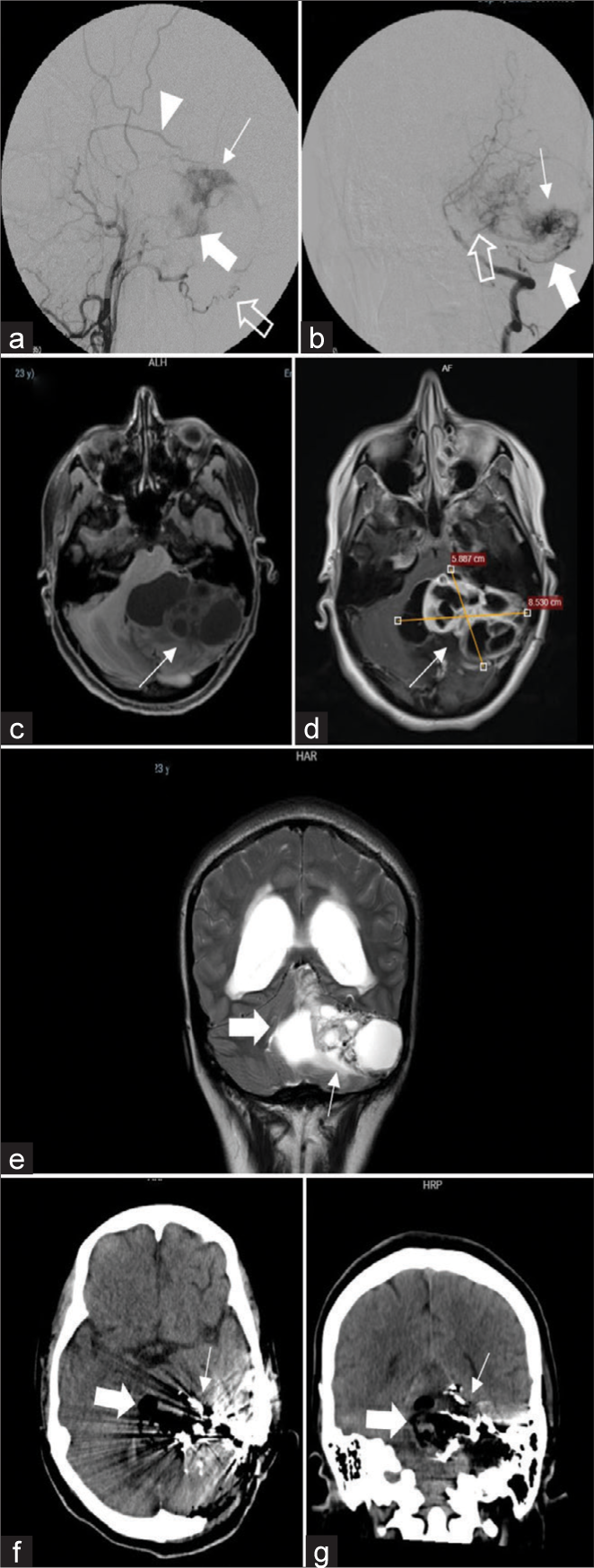
A 25-year-old woman with no significant medical history, with a previous ventricle-peritoneal shunt placement surgery and partial hemangioblastoma resection 7 years ago was admitted with a 1-year clinical history of occipital headache, nausea, and vomiting; sequelae decreased visual acuity, left dysmetria, right hemihypoesthesia and mild hemiparesis, and cerebellar ataxia. Contrast-enhanced brain magnetic resonance imaging (MRI) showed a heterogeneous tumor, with the main solid component that avidly enhances, in the left cerebellar region, 5.8 × 8.5 × 6.9 cm (AP × T × CC), which severely compresses the IV ventricle, contact the brainstem, with moderate perilesional edema [
Figure 1:
(a) Lateral incidence of angiography of the left external carotid artery, where a tumor blush is observed (thin arrow), which is irrigated through the occipital artery (empty arrow), the posterior meningeal artery (thick arrow) and the parietal branch of the left middle meningeal artery (arrowhead). (b) Anteroposterior (AP) incidence of angiography of the left vertebral artery, where it is observed the tumor blush (thin arrow), which is irrigated through the left posterior inferior cerebellar artery (thick arrow) and the left anterior inferior cerebellar artery (empty arrow). (c) Brain magnetic resonance imaging (MRI) non-enhanced T1 sequence, where a heterogeneous mass is evident, hypointense, with cystic portions in his medial part (arrow). (d) Brain MRI contrast-enhanced T1, where it is observed that the solid parts enhance (arrow), and the measurements are 5.8 × 8.5 cm (AP × T). (e) Brain MRI T2 sequence in coronal view shows a heterogeneous left cerebellar mass (thick arrow), with cystic portions and other solid, slightly hyperintense portions (thin arrow). (f and g) Immediate postoperative brain computed tomography non-enhanced in axial view (f) and coronal view (g) that shows gross total resection of the tumor that is replaced by pneumocephalus (thick arrow), it also demonstrates embolizing material in the more lateral part of the surgical area (thin arrow).
Cerebral angiography was done as part of the studies, which shows afference mostly by the parietal branch of the left middle meningeal artery (MMA) and by the posterior meningeal artery, a branch of the left ascending pharyngeal artery, and scarce filling through the left occipital artery (OA). In addition, through the vertebrobasilar system, it was seen that the tumor is irrigated by the left posterior inferior cerebellar artery (PICA) and anterior inferior cerebellar artery (AICA) [
Six days after embolization, we proceeded to perform an enlargement of the left lateral suboccipital craniectomy, dissected by plans, and a heterogeneous, fibrous, and hypervascularized lesion was found, adhered to the tentorium. It was progressively deafferented, and microsurgical dissection is done, removing the lesion en bloc, but despite the measures, considerable bleeding occurred, it did not generate instability in the intraoperative, with preoperative hemoglobin of 15.6 g/dL and postoperative of 11.5 g/dL. We had no other complications. The pathological anatomy revealed that it was a WHO grade I hemangioblastoma.
Postoperative brain tomography showed apparent gross total resection, with afferent vessels embolized in the lateral edge of the surgical field in the cerebellopontine angle cistern, with little pneumocephalus, without hematoma and other complications [
Case 2
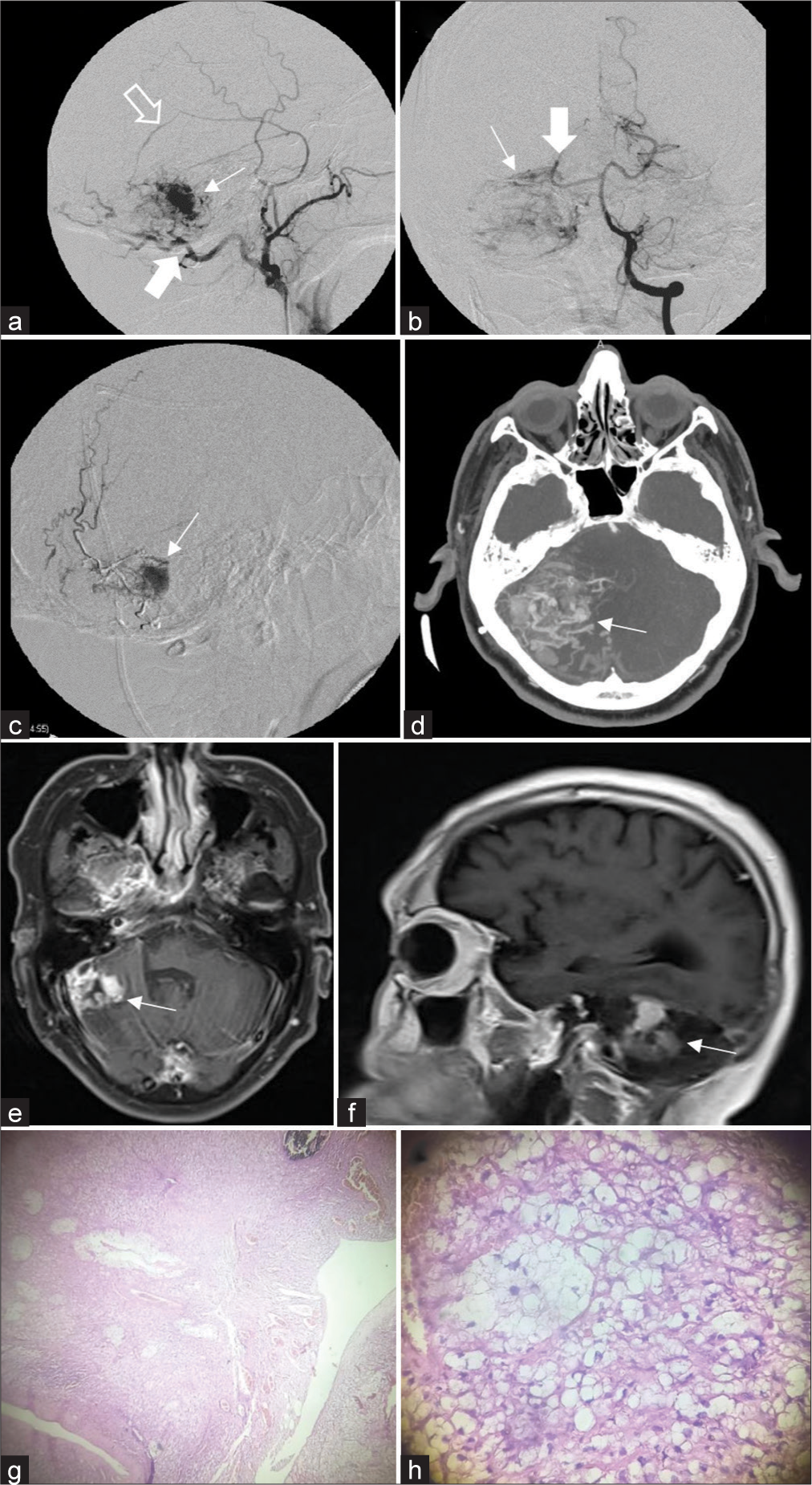
A 75-year-old male with no previous clinical records with a previous ventricle-peritoneal shunt placement surgery was admitted with a 2-year clinical history of headache, nausea, sequelae decreased visual acuity, rest tremor, and right hemiparesis. Due to this, a contrast-enhanced brain MRI was performed where a heterogeneous mass is evident, avidly enhanced, located in the right cerebellar hemisphere, 2.7 × 2.5cm (AP × T). In addition, a brain angiotomography was performed, showing multiple tumor afferents [
Figure 2:
(a) Angiography of the right external carotid artery in lateral incidence where tumor blush is evident (thin arrow) with afference through the right occipital artery (thick arrow) and the parietal branch of the right middle meningeal artery (empty arrow). (b) Left vertebral artery angiography in anteroposterior incidence where right cerebellar tumor blush is evident (thin arrow), with afference through the right superior cerebellar artery (thick arrow). (c) Intraoperative imaging during preoperative embolization, where it is observed the filling of the tumor with embolizing material (arrow). (d) Brain angiotomography in axial view shows a right cerebellar mass (arrow) that avidly enhances; it is evident that it is hypervascularized. (e and f) Contrast-enhanced brain magnetic resonance imaging T1 sequence in axial section (e) and sagittal section (f) showing recurrence of solid hemangioblastoma (arrow) in the right cerebellar hemisphere in its most lateral part. (g and h) Small nucleus cells, broad vacuolated cytoplasm, abundant vascularization with flattened endothelium and intratumoral hemorrhage areas, ×4 view (g) and ×10 view (h).
It was decided to perform a preoperative embolization. A 1.5 Fr microcatheter assisted with a 0.08 microguide is navigated through the right OA, proceeding to embolize with 3 cc of ethylene vinyl alcohol copolymer, reducing the blood flow by 90%. One week after embolization, we proceeded to perform a right lateral suboccipital craniotomy, where was evident a pearly violet tumor with afferents embolized at several levels. We proceeded to progressive deafferentation, and the tumor was resected en bloc. We had no complications. The pathological anatomy confirmed the diagnosis of the WHO grade I hemangioblastoma [
Postoperative non-enhanced brain tomography showed apparent total resection without complications in the surgical field. A contrast-enhanced brain MRI was performed 1 year after surgery, which showed a tumor recurrence of <3 Cm in the right cerebellum, compatible with a solid hemangioblastoma [
DISCUSSION
Hemangioblastomas are benign WHO grade 1 tumors [
The symptoms in more than 50% of patients are intracranial hypertension, and one-third of patients present cerebellar signs,[
They are highly vascularized tumors, although they are rarely associated with cerebral aneurysms, subarachnoid, or intratumoral hemorrhage, which was not evident in our cases either. However, Moscovici et al. found in their study a high rate of aneurysms associated with hemangioblastoma, unlike those described in the literature, hypothesizing that it could be due to flow and could resolve after tumor surgery.[
The literature mentions that solid hemangioblastomas of the posterior fossa are associated with a poor prognosis and high morbidity and mortality, reaching 50%, with even intraoperative mortality of 9%, which has been changing in recent years.[
Few studies describe the appropriate management of patients with giant posterior fossa hemangioblastomas. Still, Jeon et al. found in their study that patients with tumors of the cerebellar hemisphere and vermis had good results. Still, in those with tumors of the brainstem, treatment should be planned to achieve a near total or subtotal resection, piecemeal, if en bloc resection was not possible.[
Surgical resection is the management of choice due to its location in the posterior fossa, large size, and high vascularization, and preoperative embolization is not always a requirement.[
The surgical technique for the cystic form includes opening the cyst to expose the nodule and resecting that solid part en bloc, while in the solid form, it can be managed as an arteriovenous malformation.[
This classification used by Wan et al. was used to decide the surgical approach to be performed well. For type 1, an ipsilateral suboccipital approach is recommended; for type 2, a modified far lateral approach; for type 3, a medial suboccipital approach; and for type 4, a suboccipital supra cerebellar approach.[
Total resection of the tumor is a primary goal, leaving the drainage vein at the end of the surgery. The afferents must first be identified and progressively deafferented, then proceed with tumor resection by dissecting the peripheral tissue, ending with the ligation of the vein. Internal decompression can cause uncontrollable bleeding, which is why en-bloc resection is a good option.[
The objective of preoperative embolization is to control inaccessible afferents and, thus, reduce the vascularity of the tumor before starting surgery.[
The first reports of preoperative embolization refer to the use of polyvinyl alcohol particles between 150 to 250 um, with subsequent surgery within the 1st-week post embolization,[
CONCLUSION
Solid hemangioblastomas are less frequent than their cystic or multicystic counterpart, with their main symptoms being intracranial hypertension, in most cases due to secondary obstructive hydrocephalus, followed by cerebellar symptoms, since their most common location is the posterior fossa, and within these, the cerebellar location is the most common. The treatment is surgical resection, although it is a challenge, and it should be considered as an arteriovenous malformation, in which preoperative embolization can be used, followed by microsurgical treatment where progressive deafferentation is sought, removal of the tumor and, at the end, the closure of the drainage vein. Finally, in our experience, we had favorable results, with the patient’s clinical improvement but who showed recurrence after 2 years, despite an apparent initial total resection, which requires further studies to define additional complementary therapies such as radiotherapy.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ampie L, Choy W, Lamano JB, Kesavabhotla K, Kaur R, Parsa AT. Safety and outcomes of preoperative embolization of intracranial hemangioblastomas: A systematic review. Clin Neurol Neurosurg. 2016. 150: 143-51
2. Boutakioute B, Zouine Y, Chehboun A, Ouali M, Ganouni NC. Successful preoperative embolization of a cystic-solid variant of cerebellopontine angle hemangioblastoma. Radiol Case Rep. 2022. 17: 4799-803
3. Cui H, Zou J, Bao YH, Wang MS, Wang Y. Surgical treatment of solid hemangioblastomas of the posterior fossa: A report of 28 cases. Oncol Lett. 2017. 13: 1125-30
4. Eskridge JM, McAuliffe W, Harris B, Kim DK, Scott J, Winn HR. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol. 1996. 17: 525-31
5. Horvathy DB, Hauck EF, Ogilvy CS, Hopkins LN, Levy EI, Siddiqui AH. Complete preoperative embolization of hemangioblastoma vessels with Onyx 18. J Clin Neurosci. 2011. 18: 401-3
6. Jeon C, Choi JW, Kong DS, Nam DH, Lee JI, Seol HJ. Treatment strategy for giant solid hemangioblastomas in the posterior fossa: A retrospective review of 13 consecutive cases. World Neurosurg. 2022. 158: e214-24
7. Kamitani H, Hirano N, Takigawa H, Yokota M, Miyata H, Ohama E. Attenuation of vascularity by preoperative radiosurgery facilitates total removal of a hypervascular hemangioblastoma at the cerebello-pontine angle: Case report. Surg Neurol. 2004. 62: 238-43
8. Kuharic M, Jankovic D, Splavski B, Boop FA, Arnautovic KI. Hemangioblastomas of the posterior cranial fossa in adults: Demographics, clinical, morphologic, pathologic, surgical features, and outcomes. A systematic review. World Neurosurg. 2018. 110: e1049-62
9. Liu X, Zhang Y, Hui X, You C, Yuan F, Chen W. Surgical management of medulla oblongata hemangioblastomas in one institution: an analysis of 62 cases. Int J Clin Exp Med. 2015. 8: 5576-90
10. Matsushima T, Kawashima M, Masuoka J, Mineta T, Inoue T. Transcondylar fossa (supracondylar transjugular tubercle) approach: Anatomic basis for the approach, surgical procedures, and surgical experience. Skull Base. 2010. 20: 83-91
11. Moscovici S, Candanedo C, Spektor S, Cohen JE, Kaye AH. Solid vs. cystic predominance in posterior fossa hemangioblastomas: Implications for cerebrovascular risks and patient outcome. Acta Neurochir. 2022. 164: 1357-64
12. Rachinger J, Buslei R, Prell J, Strauss C. Solid haemangioblastomas of the CNS: A review of 17 consecutive cases. Neurosurg Rev. 2009. 32: 37-48
13. Shin GW, Jeong HW, Seo JH, Kim ST, Choo HJ, Lee SJ. Preoperative embolization of cerebellar hemangioblastoma with onyx: Report of three cases. Neurointervention. 2014. 9: 45
14. Singounas EG. Haemangioblastomas of the central nervous system. Acta Neurochir. 1978. 44: 107-13
15. Sirko A, Halkin M, Cherednychenko Y, Perepelytsia V. Staged surgical treatment of a hypervascular cerebellar hemangioblastoma and saccular superior cerebellar artery aneurysm using preoperative embolization with a low viscosity non-adhesive liquid embolic agent. Interdiscip Neurosurg. 2021. 25: 101232
16. Standard SC, Ahuja A, Livingston K, Guterman LR, Hopkins LN. Endovascular embolization and surgical excision for the treatment of cerebellar and brain stem hemangioblastomas. Surg Neurol. 1994. 41: 405-10
17. Takeuchi S, Tanaka R, Fujii Y, Abe H, Ito Y. Surgical treatment of hemangioblastomas with presurgical endovascular embolization. Neurol Med Chir (Tokyo). 2001. 41: 246-52
18. Vázquez-Añó V, Botella C, Beltrán A, Solera M, Piquer J. Preoperative embolization of solid cervicomedullary junction hemangioblastomas: Report of two cases. Neuroradiology. 1997. 39: 86-9
19. Wan J, Cui H, Wang Y. Surgical management of large solid hemangioblastomas of the posterior fossa. J Clin Neurosci. 2011. 18: 39-42
20. Young S, Richardson AE. Solid haemangioblastomas of the posterior fossa: Radiological features and results of surgery. J Neurol Neurosurg Psychiatry. 1987. 50: 155-8