- Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, United States
- Global Neurosurgical Alliance, United States,
- College of Medicine, The University of Arizona College of Medicine - Tucson, Arizona, Unite States
- Department of Neurosurgery, University of Arizona, Tucson, Arizona, Unite States,
- Department of Neurosurgery, The University of Jordan School of Medicine, Amman, Jordan,
- Department of Medicine, Aga Khan University, Karachi, Pakistan,
- Department of Neurosurgery, University of Kufa College of Medicine, Kufa, Iraq,
- Department of Medicine, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom,
- Departmenty of College of Medicine, The University of Arizona College of Medicine, Phoenix, Arizona, United States.
Correspondence Address:
Albert Alan, Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States.
DOI:10.25259/SNI_773_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Barbara Buccilli1,2, Albert Alan2,3,4, Aljeradat Baha’2,5, Akmal Shahzad2,6, Yasser F. Almealawy2,7, Nathan Simbarashe Chisvo2,8, Michelle Ennabe2,9, Martin Weinand3,4. Neuroprotection strategies in traumatic brain injury: Studying the effectiveness of different clinical approaches. 26-Jan-2024;15:29
How to cite this URL: Barbara Buccilli1,2, Albert Alan2,3,4, Aljeradat Baha’2,5, Akmal Shahzad2,6, Yasser F. Almealawy2,7, Nathan Simbarashe Chisvo2,8, Michelle Ennabe2,9, Martin Weinand3,4. Neuroprotection strategies in traumatic brain injury: Studying the effectiveness of different clinical approaches. 26-Jan-2024;15:29. Available from: https://surgicalneurologyint.com/surgicalint-articles/neuroprotection-strategies-in-traumatic-brain-injury-studying-the-effectiveness-of-different-clinical-approaches/
Abstract
Background: This review delves into clinical strategies aimed at addressing the complexities of traumatic brain injury (TBI), specifically focusing on pharmaceutical interventions and stem cell therapies as potential avenues for enhancing TBI outcomes.
Methods: A thorough review of clinical strategies for TBI management, encompassing pharmaceutical and nonpharmaceutical interventions, was performed. PubMed, MEDLINE and clinical trial databases were searched to identify relevant studies and clinical trials. Inclusion criteria consisted of studies involving pharmaceutical agents and other clinical approaches (i.e., stem cell therapies) targeting neuroinflammation, excitotoxicity, oxidative stress, and neurodegeneration in TBI. Data from clinical trials and ongoing research initiatives were analyzed to assess the current status and potential of these clinical approaches.
Results: Many trials have been conducted to face the challenge that is TBI. These interventions are designed to target critical aspects of secondary brain injury, encompassing neuroinflammation, excitotoxicity, oxidative stress, and neurodegeneration. Despite this, there is no panacea or definitive remedy for this condition. Combining therapies in a patient-tailored approach seems to be our best chance to improve these patients’ outcomes, but systematic protocols are needed.
Conclusion: Clinical strategies represent dynamic and continually evolving pathways in TBI management. This review provides an extensive overview of the existing landscape of clinical approaches and promising new studies and outlines their influence on patient outcomes. By highlighting challenges and presenting opportunities, it contributes to the ongoing mission to advance clinical care for individuals impacted by TBI.
Keywords: Excitotoxicity, Neurodegeneration, Neuroinflammation, Oxidative stress, Traumatic brain injury
INTRODUCTION
Background and significance of TBI-related neuroprotection
Traumatic brain injury (TBI) is regarded as one of the primary causes of hospitalization, disability, and death in people of all ages.[
The mechanical damage is followed by local inflammation, predominantly led by microglia.[
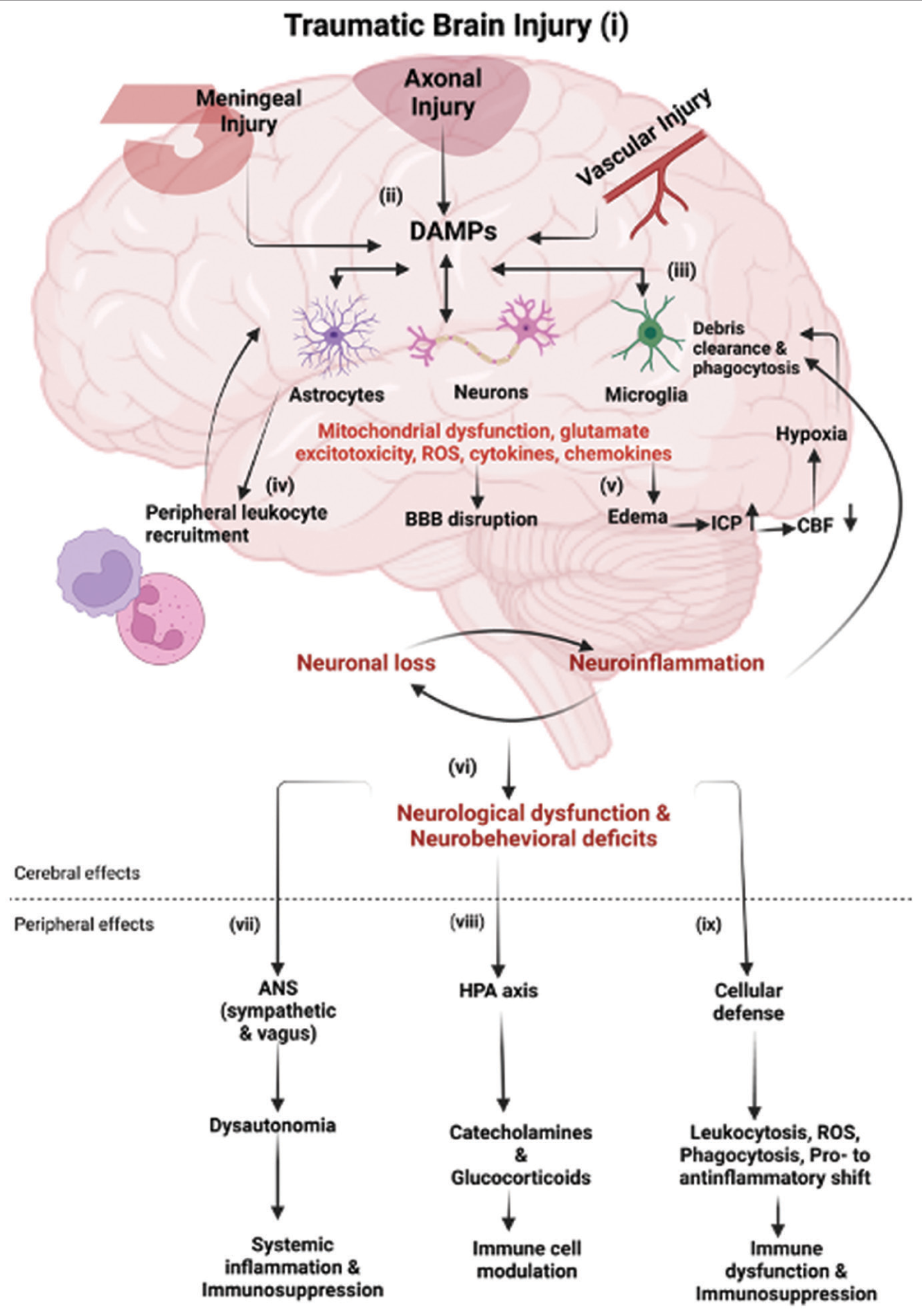
Figure 1:
Immune response following traumatic brain injury (TBI): (i-ii) following TBI, the primary mechanical injury can include meningeal contusion, axonal shearing, and cerebrovascular injury, culminating in meningeal and neuronal cell death, as well as microglial and astrocytic activation. (iii) Such neuronal injury and glial engagement generate chemokines, cytokines, and reactive oxygen species, along with the release of damage-associated molecular patterns (DAMPs), setting off an inflammatory response. (iv) In the presence of DAMPs, phagocytic microglia engage in debris clearance and synthesize neurotrophic agents. Sustained stimulation of these pathways induces subsequent injury through leukocyte recruitment, which initially aids in the removal of tissue debris. (v) Subsequently, it contributes to the progression of inflammation and disruption of the blood–brain barrier (BBB). The cytotoxic edema and compromised BBB integrity bring to an elevation of the intracranial pressure, leading to decreased cerebral blood flow, thereby intensifying hypoxia and disrupting the cerebral energy supply. Consequently, this cascade drives further neuronal depletion, propelling a self-perpetuating cycle of neuroinflammation and neurodegeneration. (vi) These progressive pathological modifications culminate in neurological dysfunction and deficits in motor, cognitive, and emotional functions. TBI also induces alterations in the autonomic nervous system (ANS), which monitors and regulates DAMPs, consequently eliciting both cerebral and peripheral immune responses. (vii) Activation of the sympathetic ANS culminates in the peripheral discharge of catecholamines (epinephrine and norepinephrine), which suppress the systemic immune responses of macrophages through the cholinergic anti-inflammatory pathway (CAO), thereby mitigating systemic inflammation. (viii) Furthermore, the release of catecholamines and glucocorticoids through the hypothalamic-pituitary-adrenal axis governs the functional behavior of systemic immune cells after TBI. (ix) The cellular immune response to traumatic brain injury involves an increase in leukocytosis and ROS generation, progresses through phagocytosis, and shifts from pro-inflammatory to anti-inflammatory states, potentially leading to immune dysfunction and immunosuppression. Abbreviations: ICP (increased intracranial pressure), CBF (cerebral blood flow), HPA (hypothalamic-pituitary-adrenal), ROS (reactive oxygen species). Image created with BioRender.com.
The rationale behind early intervention is to halt or attenuate these damaging processes before they escalate further. By doing so, early interventions have the potential to yield greater benefits in terms of preserving neural tissue, minimizing neuroinflammation, and averting excessive neuronal cell death. While the benefits of early interventions are well-established, it is important to note that the therapeutic window does not abruptly close as time progresses. Some interventions can still be efficacious when introduced later in the recovery process. This is particularly relevant in cases where the secondary injury processes persist over an extended period. Late-stage interventions may focus on facilitating neural repair, enhancing cognitive rehabilitation, or addressing chronic neurological deficits.
TBI’s social and financial implications are presently attracting an increasing amount of attention.[
Overview of TBI epidemiology and impact on the brain
Age and gender both affect the incidence of TBI. It can occur in children as young as 0 years old, teenagers as old as 15–19, and adults. The incidence is higher in men than women.[
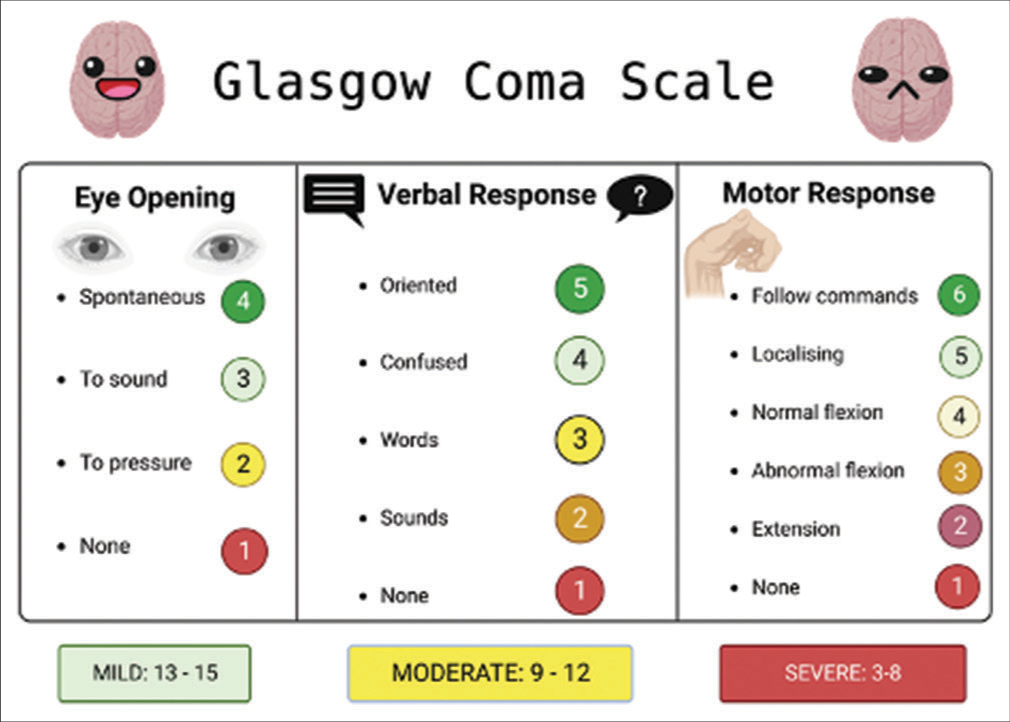
Figure 2:
This figure illustrates the Glasgow coma scale (GCS), a vital neurological assessment tool, as it pertains to traumatic brain injury (TBI). The GCS quantifies the patient’s level of consciousness based on eye, verbal, and motor responses, aiding clinicians in gauging TBI severity and guiding treatment decisions. Created with BioRender.com.
Mild TBI (mTBI), the most prevalent kind of TBI (representing 70% of cases), has good survival rates, but up to 5 million Americans live with persistent TBI-related disabilities, including social, motor and cognitive dysfunctions, mood disorders, sleep disturbances, and personality changes.[
The primary injury in TBI can lead to focal and diffuse brain damage, which often coexist in moderate-to-severe cases.[
The secondary damage is frequently delayed and protracted, and multiple variables, including excitotoxicity, mitochondrial dysfunction, oxidative stress, lipid peroxidation, neuroinflammation, axon degeneration, and apoptosis, play a role in it.[
24 h post-TBI, the BBB shows signs of malfunctioning, allowing circulating leukocytes to infiltrate the damaged brain parenchyma and release proinflammatory cytokines such as Interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNFa), and complement factors.[
A patient might experience accelerated neurodegeneration and the onset of chronic traumatic encephalopathy following a single or recurrent TBI. This has been seen in athletes and military personnel exposed to frequent head trauma and concussions.[
An investigation into the connection between TBI and neurodegenerative conditions found an association linking TBI and Alzheimer’s. The findings showed that TBI causes tau protein to be acetylated, a mechanism connected to Alzheimer’s. In animal’s memory, problems were linked to this acetylation, demonstrating the long-term effects of TBI.[
Scope and objectives of the review
The purpose of this review is to extensively examine and assess various clinical strategies for neuroprotection in the context of TBI. It is crucial to comprehend how different neuroprotective methods and treatments can be effective in reducing the impact of TBI and enhancing patient outcomes. This review intends to cover a broad spectrum of neuroprotective approaches, encompassing pharmaceutical treatments, natural compounds, as well as cellular and molecular methods. Through a thorough examination of existing literature and clinical trials, the review aims to provide a comprehensive overview of the strengths and weaknesses of each neuroprotective strategy and its potential for practical application in clinical settings. In essence, the goals of this review are to offer valuable insights to healthcare professionals, researchers, and policymakers regarding evidence-based neuroprotection techniques that show promise in mitigating the consequences of TBI and promoting improved patient recovery and quality of life.
METHODOLOGY
Inclusion and exclusion criteria for article selection
Inclusion and exclusion criteria play a crucial role in ensuring that the articles selected for this review are relevant and meet the objectives of the study. The following are the inclusion and exclusion criteria established for article selection. We included articles that directly address neuroprotection strategies in the context of TBI and focus on the prevention, reduction, or mitigation of brain injury after a TBI event. Articles published within the past 20 years have been given priority to ensure that the review reflects current research and developments in the field. Both preclinical and clinical studies have been included. This encompasses animal studies, in vitro experiments, as well as randomized controlled trials (RCTs), cohort studies,case– control studies, and systematic reviews.
Only articles written in English have been included for ease of comprehension and analysis, and articles accessible through academic databases, online journals, and reputable sources have been prioritized to ensure reliability and credibility.
We excluded articles not directly related to neuroprotection in the context of TBI. This includes studies focused solely on other brain disorders or general neurological conditions. Gray literature, conference abstracts, editorials, opinions, and non-peer-reviewed articles have been excluded due to potential limitations in the rigor and credibility of the information presented, and articles not written in English have been excluded to avoid translation-related inaccuracies. In case of duplicate publications, only the most comprehensive and recent version has been included to avoid redundancy.
By adhering to these inclusion and exclusion criteria, the review aims to maintain a high standard of academic rigor, relevance, and reliability. The selected articles will contribute to a comprehensive and evidence-based analysis of neuroprotection strategies in the context of TBI, enabling a meaningful synthesis of findings and implications for clinical practice and future research.
Search strategy and databases used
We identified relevant keywords and phrases related to the topic, including “traumatic brain injury,” “TBI,” “neuroprotection,” “neuroprotective agents,” “interventions,” “clinical trials,” and “brain injury outcome.” We then combined these keywords using Boolean operators (AND, OR) to formulate effective search strings. For example, we used: (Traumatic brain injury OR TBI) AND (neuroprotection OR neuroprotective agents), (neuroprotection OR neuroprotective interventions) AND (brain injury outcome OR clinical trials), and (TBI, Neuroprotection, Outcomes evaluation, biomarkers, Imaging techniques, Challenges, “Brain Injuries, Traumatic”[Mesh], “Neuroprotection”[Mesh], “Outcome Assessment, Health Care”[Mesh], “Biomarkers”[Mesh], and “Diagnostic Imaging”[Mesh]). We incorporated synonyms, alternate spellings, and related terms to capture a wider range of relevant articles. Our search spanned the following databases: PubMed, Embase/MEDLINE, and Scopus.
To ensure a thorough search, the reference lists of relevant review articles and included studies have been manually checked for potentially relevant articles that may not have appeared in the initial database search.
By employing this comprehensive search strategy and using reputable databases, the review aims to gather a diverse and extensive collection of literature on neuroprotection strategies in TBI, enabling a robust analysis and synthesis of the available evidence.
PHARMACEUTICAL INTERVENTIONS
A recent study developed bioactive nanofibrous dural substitutes that release insulin-like growth factor 1 (IGF-1). These substitutes significantly enhanced neural cell survival post-TBI.[
A pediatric preclinical study on the effects of LM22A-4, a TrkB agonist, revealed that treated animals displayed improved anxiety-related behavior.[
Another approach combined ketamine and perampanel to study their effects on TBI-induced behavioral changes in mice.[
Numerous studies have explored the potential neuroprotective properties of erythropoietin (EPO) in the context of TBI. EPO’s mechanism of action involves binding to the EPO receptor, which leads to downstream signaling activation. This includes JAK-2 phosphorylation, initiating pathways such as PI3-K/Akt, Ras/MAPK, and JAK2-STAT, which are pivotal for EPO’s antiapoptotic and trophic effects. In addition, EPO has been shown to mitigate excitotoxicity, oxidative stress, and inflammation. It enhances neuronal viability under stress conditions and reduces glutamate toxicity, primarily through calcium-dependent mechanisms. Furthermore, EPO potentially improves cerebral perfusion and vascular integrity and stimulates angiogenesis through the VEGF pathway. It also plays a role in neurogenesis following TBI. EPO’s hematopoietic side effects have prompted the development of EPO analogs with neuroprotective potential. Clinical trials have yielded mixed results, with some suggesting neuroprotective effects while others remain inconclusive. Further research is needed to establish EPO’s definitive role in TBI treatment.[
Progesterone is another drug with neuroprotective capabilities which uses a series of intracellular pathways to achieve its effect. One study shows how EGFR activation mitigates BBB cell connexin loss in brain injury, stabilizing BBB structure, decreasing permeability, and reducing brain tissue edema. ERK, a MAPK family member encoded by the MAPK1 gene, operates through the Ras/Raf/MEK/ERK1/2 pathway.[
Progesterone treatment in TBI influences steroid hormone receptor activity, RNA polymerase II transcription factor activity, receptor signaling protein tyrosine kinase activity, MAP kinase, and related protein kinase activity. KEGG analysis reveals core targets of progesterone treatment regulating apoptosis and signaling transduction. These targets enrich PI3K/Akt, Ras, and MAPK pathways, indicating multi-pathway, multi-target effects for TBI treatment.[
The MAPK pathway, a prominent KEGG finding, employs a 3-stage enzymatic cascade (MAP3K/MAP2K/MAPK) to activate downstream transcription factors for signal transduction. JNK, a MAPK family component, participates in extracellular stimulus-induced activities such as apoptosis, metabolism, and DNA repair. In TBI, downregulated JNK3 expression aids nerve function recovery and reduces edema and nerve cell apoptosis through TNFa, IL-1a, IL-1b, and IL-6 downregulation.[
P38 MAPK, a vital serine/threonine protein kinase, influences neural tissue pathophysiology. This pathway responds to oxidative stress and inflammation, initially inducing neurotoxicity and later inhibiting inflammation through an anti-apoptotic effect. P38 MAPK regulates apoptosis-related protein expression and enhances BBB permeability.[
Ras signaling, a classical MAPK pathway, operates through the Ras/Raf/MEK/ERK1/2 route. Inhibiting ERK/MAPK reduces neuronal injury, apoptosis, brain edema, and neurological damage. EGFR interaction with ligands like EGF activates Ras signaling, protecting against ischemic stroke and inflammation while promoting neuroprotection through IL-20 and reduced excitatory amino acid release.
Thus, progesterone’s influence on MAPK and Ras pathways mitigates secondary TBI effects and neuronal apoptosis and enhances neurological function.[
Numerous investigations have demonstrated the neuroprotective attributes of progesterone in both animal models and clinical trials of TBI, with no reports of severe adverse effects associated with treatment.[
However, the outcomes of two randomized, double-masked, and multicenter Phase III RCTs have indicated no significant differences between placebo- and progesterone-treated groups concerning mortality rates and functional outcomes at the 6-month post-TBI mark.[
Another study included seven RCTs that were also featured in the meta-analysis. While these trials spanned follow-up periods ranging from 30 days to 6 months, they did not conduct stratified analyses based on follow-up duration, overlooking potential time-dependent effects of progesterone. The researcher’s investigation assessed the efficacy of progesterone in severe TBI patients at both short-term (within three months postinjury) and long-term (at six months postinjury) endpoints. Remarkably, progesterone administration correlated with a reduced mortality rate and higher GOS scores within the first three months following injury; however, no significant advantages were observed at the 6-month mark. It is important to note that interpreting the long-term effects of progesterone should be approached with caution due to the influence of numerous confounding factors.[
In summary, progesterone administration enhances clinical outcomes in severe TBI patients within three months following injury, although discernible long-term benefits at the 6-month postinjury interval are less evident. Further, investigations are warranted to thoroughly explore inter-study variations and inform the design of future clinical trials.
N-acetylcysteine (NAC) is a compound known for its antioxidant properties and its potential as a neuroprotective agent.[
N-acetyl cysteine has demonstrated significant neuroprotective effects in animal models, particularly in mitigating secondary neuronal injury following TBI. Studies in rats have confirmed the beneficial antioxidant effects of NAC when administered after brain injury.[
A double-masked and placebo-controlled clinical trial was conducted to assess the efficacy of NAC in patients with mTBI caused by blasts. The treatment group received 2 g of NAC twice daily for the first four days, followed by 1.5 g of NAC twice daily for the next three days. After seven days of treatment, patients were evaluated for symptoms such as dizziness, headache, hearing loss, memory loss, sleep disturbances, and neurocognitive dysfunction. Significant improvements (P < 0.01) in these symptoms were observed in patients who received NAC within 24 hours of injury, and the treatment group had an 86% chance of recovery. These findings suggest the need for further investigation into the long-term effects of NAC treatment in TBI.[
In addition, in 2017, Clark et al. conducted a randomized, double-masked, and placebo-controlled Phase I study in children aged 2–18 who were admitted to a Pediatric Intensive Care Unit after severe TBI.[
TARGETING INFLAMMATION AND OXIDATIVE STRESS
The impact of inflammation and oxidative stress in TBI
According to normal physiology, both intracellular and extracellular processes contribute to and lead to secondary injury. Inflammatory and oxidative responses following TBI can be disruptive. Proinflammatory factors such as tumor necrosis factor and IL -6, along with reactive species such as superoxide and peroxynitrite, are found in high concentrations in injured brain tissue. All these factors can contribute to increased inflammation, leading to damage to neuronal tissue and DNA, particularly due to reactive species. However, this also promotes apoptosis of the affected cells.[
Anti-inflammatory and antioxidant therapies explored
NMDA-receptor antagonists
Elevated concentrations of glutamate exceeding 100 μM are implicated in neuronal destruction and cell death.[
Glutamate agonists
Microglia, astrocytes, and neurons exhibit high glutamate receptor expression. Glutamate agonists have been observed to inhibit caspase-dependent apoptosis and mitigate microglial inhibition of NADPH oxidase. The mGluR5 agonist (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) has demonstrated neuroprotective and anti-apoptotic properties in neuronal and microglial cultures. In summary, early treatment with glutamate agonists in laboratory settings has shown promising neuroprotective effects post-TBI.[
Calcium-channel antagonists
The blockade of neural calcium channels holds the potential to mitigate glutamate excitotoxicity, reduce neurotransmitter release, and disrupt the apoptosis cascade. Calcium channel blockers such as nicardipine and nimodipine have been suggested to play a neuroprotective role and mitigate vasospasm in subarachnoid hemorrhage.[
Immune system modulation
The immunosuppressant Cyclosporine-A reduces T-cell-mediated immunity and is used in organ transplant recipients. It inhibits calcineurin and cyclophilin-A, suppressing mitochondrial pore formation and potentially curbing the apoptotic cascade.[
Challenges and potential for clinical translation
On the frontier of data-driven research, Lipponen et al. developed a unique pipeline for TBI treatment discovery using transcriptomics data.[
A recent study focused on the role of NADPH oxidase 2 (NOX2) in TBI. The outcomes showed that GSK2795039 reduced NOX2 expression and activity in a TBI mouse model. In addition, treated mice displayed improved cognitive functions, suggesting that targeting NOX2 could be a potential therapeutic strategy.[
Therapeutic hypothermia and brain cooling
Hypothermia was first described in the Edwin Smith Papyrus, an ancient Egyptian treatise on medicine and surgery written over 5000 years ago.[
CELLULAR AND MOLECULAR APPROACHES
Cell-based therapies and gene therapies for TBI neuroprotection
A recent study aimed to evaluate the effects of administering a single dose of exosomes early after injury over seven days in a swine model of TBI and hemorrhagic shock. At the end of the seven days postinjury, levels of markers related to inflammation, apoptosis, and neural plasticity were analyzed.[
A recent study delved into the impact of intramuscular IGF-1 gene therapy.[
Another study explored stem cell therapy combined with genetic modifications. The outcomes showed that rats treated with mesenchymal stem cells overexpressing IL-10 had reduced autophagy response, suggesting enhanced neural protection.[
CLINICAL IMPLICATIONS AND CHALLENGES
Translating neuroprotection strategies to clinical practice
Facilitating the transition of experimental neuroprotection methods into practical clinical applications represents a pivotal step in advancing TBI care. This phase demands meticulous planning and comprehensive evaluation to integrate these strategies seamlessly into established clinical protocols. The employment of neuroprotection techniques requires the establishment of standardized guidelines and procedures that harmonize with current therapeutic approaches. Extensive clinical trials and real-world investigations are imperative to ascertain the safety, efficacy, and feasibility of these therapies and bridge the divide between laboratory research and clinical practice. Collaboration among neurologists, neurosurgeons, rehabilitation specialists, and other medical experts is essential, fostering a coordinated approach that optimizes patient care and capitalizes on the potential benefits of neuroprotection methods.
Challenges in implementation and personalized approaches
The effective implementation of neuroprotection techniques encounters several challenges despite their considerable potential. Patient variability, injury severity, and individual responses to treatment underscore the importance of tailored strategies for each TBI case. Overcoming logistical hurdles, securing funding, and educating healthcare professionals are essential steps for integrating neuroprotection methods across diverse clinical settings. The adoption of innovative interventions demands a delicate balance between the need for swift deployment of effective therapies and the thorough evaluation required for their safe and successful application. The development of adaptable and scalable procedures that consider the complex interplay of clinical, logistical, and patient-related factors is pivotal in surmounting these obstacles.
ICP and cerebral perfusion pressure (CPP) shed light on the dynamics of CBF. However, using probes like PbtO2, which measures extracellular oxygen tension, metabolic events can actually be measured.[
Defining optimal PbtO2 target values is complex.[
Metabolic crisis can be recognized by a high lactate: pyruvate ratio, which also serves as a standalone predictor of death.[
The idea of employing advanced multimodal monitoring to guide the treatment of elderly patients is appealing, but there exists a notable gap in our understanding of this domain. This knowledge deficit can be attributed in part to the elevated risks linked with invasive intracranial monitoring in older individuals. Many of these patients are on anticoagulant and antiplatelet medications, which heighten the potential for complications. Furthermore, due to the possibility of a less-than-optimal outcome, there is a reduced inclination toward intensive monitoring and treatment in this demographic.
Ethical considerations
Integrating neuroprotection techniques into TBI treatment demands careful ethical considerations. This involves risk assessment, informed consent, and addressing cost and access issues for equitable treatment. Patient, caregiver, and family perspectives are vital. Engaging with TBI patients’ experiences guides ethical decision-making and enhances the practicality of neuroprotection techniques. A comprehensive framework balancing beneficence, autonomy, and justice emerges through the convergence of ethics and patient insights.
CONCLUSION
This comprehensive review delves into various neuroprotective strategies for TBI. These interventions work through intricate molecular pathways, affecting processes such as apoptosis, inflammation, oxidative stress, and excitotoxicity. Clinical trials have produced mixed results, influenced by factors such as administration methods, dosages, and follow-up durations.
TBI management proved to be extremely complex since no single intervention is a panacea for this multifaceted condition. Instead, a holistic approach considering patient-specific factors, timing, and a combination of therapies is crucial for improving outcomes. Ongoing efforts to standardize protocols and refine patient selection criteria offer the promise of more reliable future treatments. Despite challenges, the pursuit of neuroprotective strategies in TBI offers hope for better patient outcomes. The evolving landscape of TBI research holds the potential for continued progress in understanding and managing this critical public health concern.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alam S, Liu Q, Liu S, Liu Y, Zhang Y, Yang X. Up-regulated cathepsin C induces macrophage M1 polarization through FAK-triggered p38 MAPK/NF-kB pathway. Exp Cell Res. 2019. 382: 111472
2. Alqahtani F, Assiri MA, Mohany M, Imran I, Javaid S, Rasool MF. Coadministration of ketamine and perampanel improves behavioral function and reduces inflammation in acute traumatic brain injury mouse model. Biomed Res Int. 2020. 2020: 3193725
3. Aminmansour B, Fard SA, Habibabadi MR, Moein P, Norouzi R, Naderan M. The efficacy of cyclosporine-A on diffuse axonal injury after traumatic brain injury. Adv Biomed Res. 2014. 3: 35
4. Britton KR. Case study: Medroxyprogesterone in the treatment of aggressive hypersexual behaviour in traumatic brain injury. Brain Inj. 1998. 12: 703-7
5. Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: Effects of moderate hypothermia. J Neurotrauma. 2007. 24: 1707-17
6. Candolfi M, Jaita G, Zaldivar V, Zárate S, Ferrari L, Pisera D. Progesterone antagonizes the permissive action of estradiol on tumor necrosis factor-a-induced apoptosis of anterior pituitary cells. Endocrinology. 2005. 146: 736-43
7. Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004. 43: 28-60
8. Chen G, Shi J, Hu Z, Hang C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: A potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008. 2008: 716458
9. Cheng CY, Ho TY, Hsiang CY, Tang NY, Hsieh CL, Kao ST. Angelica sinensis exerts angiogenic and anti-apoptotic effects against cerebral ischemia-reperfusion injury by activating p38MAPK/HIF-1a/VEGF-a signaling in rats. Am J Chin Med. 2017. 45: 1683-708
10. Cheng CY, Tang NY, Kao ST, Hsieh CL. Ferulic acid administered at various time points protects against cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemiareperfusion injury in rats. PLoS One. 2016. 11: e0155748
11. Clark RSB, Empey PE, Bayır H, Rosario BL, Poloyac SM, Kochanek PM. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PloS one. 2017. 12: e0180280
12. Cook AM, Whitlow J, Hatton J, Young B. Cyclosporine A for neuroprotection: Establishing dosing guidelines for safe and effective use. Expert Opin Drug Saf. 2009. 8: 411-9
13. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015. 72: 355-62
14. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019. 130: 1080-97
15. Ellis EF, Dodson LY, Police RJ. Restoration of cerebrovascular responsiveness to hyperventilation by the oxygen radical scavenger n-acetylcysteine following experimental traumatic brain injury. J Neurosurg. 1991. 75: 774-9
16. Enriquez P, Bullock R. Molecular and cellular mechanisms in the pathophysiology of severe head injury. Curr Pharm Des. 2004. 10: 2131-43
17. Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E. The antioxidants a-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003. 84: 1173-83
18. Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015. 127: 3-13
19. Faul M, Wald MM, Xu L, Coronado VG, editors. Traumatic brain injury in the United States; Emergency department visits, hospitalizations, and deaths, 2002-2006. United States: National Center for Injury Prevention and Control; 2010. p.
20. Feigin VL, Theadom A, Barker-Collo S, Starkey NJ, McPherson K, Kahan M. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013. 12: 53-64
21. Fletcher JL, Dill LK, Wood RJ, Wang S, Robertson K, Murray SS. Acute treatment with TrkB agonist LM22A-4 confers neuroprotection and preserves myelin integrity in a mouse model of pediatric traumatic brain injury. Exp Neurol. 2021. 339: 113652
22. Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010. 27: 497-507
23. Giustarini D, Milzani A, Dalle-Donne I, Tsikas D, Rossi R. N-Acetylcysteine ethyl ester (NACET): A novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem Pharmacol. 2012. 84: 1522-33
24. Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012. 4: 134ra60
25. Goodman JC, Van M, Gopinath SP, Robertson CS. Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl. 2008. 102: 437-9
26. Hall ED, Andrus PK, Yonkers PA. Brain hydroxyl radical generation in acute experimental head injury. J Neurochem. 1993. 60: 588-94
27. Hanscom M, Loane DJ, Shea-Donohue T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest. 2021. 131: e143777
28. Hatton J, Rosbolt B, Empey P, Kryscio R, Young B. Dosing and safety of cyclosporine in patients with severe brain injury. J Neurosurg. 2008. 109: 699-707
29. Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: A phase II randomized control trial. J Cereb Blood Flow Metab. 2014. 34: 845-51
30. Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020. 40: 2960-74
31. Herrera ML, Bandín S, Champarini LG, Hereñú CB, Bellini MJ. Intramuscular insulin-like growth factor-1 gene therapy modulates reactive microglia after traumatic brain injury. Brain Res Bull. 2021. 175: 196-204
32. Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: A double-blind, placebo controlled study. PLoS One. 2013. 8: e54163
33. Iankova A. The glasgow coma scale: Clinical application in emergency departments. Emerg Nurse. 2006. 14: 30-5
34. Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury?. Lancet Neurol. 2002. 1: 383-6
35. James SL, Theadom A, Ellenbogen RG, Bannick MS, MontjoyVenning W, Lucchesi LR. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. 18: 56-87
36. Keating CE, Cullen DK. Mechanosensation in traumatic brain injury. Neurobiol Dis. 2021. 148: 105210
37. Kiening KL, Unterberg AW, Bardt TF, Schneider GH, Lanksch WR. Monitoring of cerebral oxygenation in patients with severe head injuries: Brain tissue PO2 versus jugular vein oxygen saturation. J Neurosurg. 1996. 85: 751-7
38. Kim CC, Nakamura MC, Hsieh CL. Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation. 2016. 13: 117
39. Kossmann T, Stahel PF, Lenzlinger PM, Redl H, Dubs RW, Trentz O. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J Cereb Blood Flow Metab. 1997. 17: 280-9
40. Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J. Incidence of traumatic brain injury across the full disease spectrum: A population-based medical record review study. Epidemiology. 2011. 22: 836-44
41. Levin SW, Baker EH, Zein WM, Zhang Z, Quezado ZM, Miao N. Oral cysteamine bitartrate and N-acetylcysteine for patients with infantile neuronal ceroid lipofuscinosis: A pilot study. Lancet Neurol. 2014. 13: 777-87
42. Li X, Xie Z, Zhou Q, Tan X, Meng W, Pang Y, editors. Glymphatic improves inammation and apoptosis after cerebral ischemiareperfusion injury in mice through ERK signaling pathway. Mol Neurobiol. 2023. p.
43. Lipponen A, Natunen T, Hujo M, Ciszek R, Hämäläinen E, Tohka J. In vitro and in vivo pipeline for validation of disease-modifying effects of systems biology-derived network treatments for traumatic brain injury-lessons learned. Int J Mol Sci. 2019. 20: 5395
44. Liu C, Huang C, Xie J, Li H, Hong M, Chen X. Potential efficacy of erythropoietin on reducing the risk of mortality in patients with traumatic brain injury: A systematic review and meta-analysis. Biomed Res Int. 2020. 2020: 7563868
45. Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010. 31: 596-604
46. Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of post-traumatic hypothermia. J Neurotrauma. 2009. 26: 1123-34
47. Lu XY, Sun H, Li QY, Lu PS. Progesterone for traumatic brain injury: A meta-analysis review of randomized controlled trials. World Neurosurg. 2016. 90: 199-210
48. Maas AI, Fleckenstein W, Jong DA, Santbrink H, editors. Monitoring cerebral oxygenation: Experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Monitoring of cerebral blood flow and metabolism in intensive care. Vienna: Springer Vienna; 1993. p. 50-7
49. Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: Past experience and current developments. Neurotherapeutics. 2010. 7: 115-26
50. Magnoni S, Ghisoni L, Locatelli M, Caimi M, Colombo A, Valeriani V. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: A microdialysis study. J Neurosurg. 2003. 98: 952-8
51. Maiti P, Peruzzaro S, Kolli N, Andrews M, Al-Gharaibeh A, Rossignol J. Transplantation of mesenchymal stem cells overexpressing interleukin-10 induces autophagy response and promotes neuroprotection in a rat model of TBI. J Cell Mol Med. 2019. 23: 5211-24
52. Martínez Banaclocha M. N-acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: Implications for treatment of Parkinson’s disease. Brain Res. 2000. 859: 173-5
53. McConeghy KW, Hatton J, Hughes L, Cook AM. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012. 26: 613-36
54. McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009. 68: 709-35
55. McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013. 136: 43-64
56. Meixensberger J, Jaeger M, Väth A, Dings J, Kunze E, Roosen K. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003. 74: 760-4
57. Menon DK, Coles JP, Gupta AK, Fryer TD, Smielewski P, Chatfield DA. Diffusion limited oxygen delivery following head injury. Crit Care Med. 2004. 32: 1384-90
58. Meyfroidt G, Taccone FS. Another failed attempt of neuroprotection: Progesterone for moderate and severe traumatic brain injury. Minerva Anestesiol. 2016. 82: 486-91
59. Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr Opin Crit Care. 2002. 8: 101-5
60. Nangunoori R, Maloney-Wilensky E, Stiefel M, Park S, Andrew Kofke W, Levine JM. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: A systematic literature review. Neurocrit Care. 2012. 17: 131-8
61. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
62. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD. The international incidence of traumatic brain injury: A systematic review and meta-analysis. Can J Neurol Sci. 2016. 43: 774-85
63. Nortje J, Coles JP, Timofeev I, Fryer TD, Aigbirhio FI, Smielewski P. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: Preliminary findings. Crit Care Med. 2008. 36: 273-81
64. Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2020. 2: CD004863
65. Pan ZY, Zhao YH, Huang WH, Xiao ZZ, Li ZQ. Effect of progesterone administration on the prognosis of patients with severe traumatic brain injury: A meta-analysis of randomized clinical trials. Drug Des Devel Ther. 2019. 13: 265-73
66. Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015. 157: 1683-96
67. Peng Y, Zhao Y, Huang Y, Liu X, Zhang H, Zhao Z. Neuroprotective effects of low-intensity transcranial ultrasound stimulation combined with Baicalin intervention on traumatic brain injury in animals. Brain Res Bull. 2021. 175: 246-53
68. Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: The assessment of normal values. J Neurotrauma. 2008. 25: 1173-7
69. Petersen A, Soderstrom M, Saha B, Sharma P. Animal models of traumatic brain injury: A review of pathophysiology to biomarkers and treatments. Exp Brain Res. 2021. 239: 2939-50
70. Ponce LL, Navarro JC, Ahmed O, Robertson CS. Erythropoietin neuroprotection with traumatic brain injury. Pathophysiology. 2013. 20: 31-8
71. Quintard H, Patet C, Zerlauth JB, Suys T, Bouzat P, Pellerin L. Improvement of neuroenergetics by hypertonic lactate therapy in patients with traumatic brain injury is dependent on baseline cerebral lactate/pyruvate ratio. J Neurotrauma. 2016. 33: 681-7
72. Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002. 17: 1137-52
73. Reinert M, Schaller B, Widmer HR, Seiler R, Bullock R. Influence of oxygen therapy on glucose-lactate metabolism after diffuse brain injury. J Neurosurg. 2004. 101: 323-9
74. Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994. 129: 64-9
75. Rosenthal G, Hemphill JC, Sorani M, Martin C, Morabito D, Obrist WD. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2008. 36: 1917-24
76. Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008. 25: 719-38
77. Sahuquillo J, Vilalta A. Cooling the injured brain: How does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007. 13: 2310-22
78. Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013. 1830: 4117-29
79. Santarsieri M, Niyonkuru C, Mccullough EH, Dobos JA, Dixon CE, Berga SL. Cerebrospinal fluid cortisol and progesterone profiles and outcomes prognostication after severe traumatic brain injury. J Neurotrauma. 2014. 31: 699-712
80. Sarrafzadeh AS, Kiening KL, Bardt TF, Schneider GH, Unterberg AW, Lanksch WR, editors. Cerebral oxygenation in contusioned vs. Nonlesioned brain tissue: Moniting of PtiO2 with licox and paratrend. Intracranial pressure and neuromonitoring in brain injury. Vienna: Springer Vienna; 1998. p. 186-9
81. Schmidt OI, Infanger M, Heyde CE, Ertel W, Stahel PF. The role of neuroinflammation in traumatic brain injury. Eur J Trauma. 2004. 30: 135-49
82. Schober ME, Block B, Beachy JC, Statler KD, Giza CC, Lane RH. Early and sustained increase in the expression of hippocampal IGF-1, but not EPO, in a developmental rodent model of traumatic brain injury. J Neurotrauma. 2010. 27: 2011-20
83. Shin MK, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K, Cintrón-Pérez CJ. Reducing acetylated tau is neuroprotective in brain injury. Cell. 2021. 184: 2715-32.e23
84. Shohami E, Beit-Yannai E, Horowitz M, Kohen R. Oxidative stress in closed-head injury: Brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab. 1997. 17: 1007-19
85. Singh SP, Jadhav SH, Chaturvedi CP, Nityanand S. Therapeutic efficacy of multipotent adult progenitor cells versus mesenchymal stem cells in experimental autoimmune encephalomyelitis. Regen Med. 2017. 12: 377-96
86. Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: A cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010. 113: 556-63
87. Spiotta AM, Stiefel MF, Gracias VH, Garuffe AM, Kofke WA, Maloney-Wilensky E. Brain tissue oxygen-directed management and outcome in patients with severe traumatic brain injury. J Neurosurg. 2010. 113: 571-80
88. Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death?. J Neurosci Res. 2005. 79: 231-9
89. Te Ao B, Brown P, Tobias M, Ameratunga S, Barker-Collo S, Theadom A. Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology. 2014. 83: 1645-52
90. Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 1976. 34: 45-55
91. Thatcher JD. The Ras-MAPK signal transduction pathway. Sci Signal. 2010. 3: tr1
92. Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O’Connell MT, Czosnyka M. Cerebral extracellular chemistry and outcome following traumatic brain injury: A microdialysis study of 223 patients. Brain. 2011. 134: 484-94
93. Urbano LA, Oddo M. Therapeutic hypothermia for traumatic brain injury. Curr Neurol Neurosci Rep. 2012. 12: 580-91
94. Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998. 26: 1576-81
95. van Santbrink H, Maas AI, Avezaat CJ. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996. 38: 21-31
96. Veenith TV, Carter EL, Grossac J, Newcombe VF, Outtrim JG, Nallapareddy S. Use of diffusion tensor imaging to assess the impact of normobaric hyperoxia within at-risk pericontusional tissue after traumatic brain injury. J Cereb Blood Flow Metab. 2014. 34: 1622-7
97. Vink R, Van Den Heuvel C. Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin Investig Drugs. 2004. 13: 1263-74
98. Wang H, Olivero W, Wang D, Lanzino G. Cold as a therapeutic agent. Acta Neurochir (Wien). 2006. 148: 565-70
99. Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008. 28: 1361-8
100. Wang M, Luo L. An effective NADPH oxidase 2 inhibitor provides neuroprotection and improves functional outcomes in animal model of traumatic brain injury. Neurochem Res. 2020. 45: 1097-106
101. Wang X, Dai Y, Zhao Y, Li M, Zhang J, Ci Y. AnnexinA5 Might suppress the phenotype of human gastric cancer cells via ERK pathway. Front Oncol. 2021. 11: 665105
102. Wang Y, Guo Q, Wang W, Wang Y, Fang K, Wan Q. Potential use of bioactive nanofibrous dural substitutes with controlled release of IGF-1 for neuroprotection after traumatic brain injury. Nanoscale. 2022. 14: 18217-30
103. Wiest DB, Chang E, Fanning D, Garner S, Cox T, Jenkins DD. Antenatal pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J Pediatr. 2014. 165: 672-7.e2
104. Williams AM, Wu Z, Bhatti UF, Biesterveld BE, Kemp MT, Wakam GK. Early single-dose exosome treatment improves neurologic outcomes in a 7-day swine model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2020. 89: 388-96
105. Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017. 16: 813-25
106. Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007. 49: 391-402.e2
107. Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007. 12: 1365-75
108. Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit Care. 2008. 12: R61
109. Xiong Y, Peterson PL, Lee CP. Effect of N-acetylcysteine on mitochondrial function following traumatic brain injury in rats. J Neurotrauma. 1999. 16: 1067-82
110. Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: Potential signaling mechanisms. J Neurosci Res. 2006. 83: 1241-51
111. Zhang F, Wang S, Cao G, Gao Y, Chen J. Signal transducers and activators of transcription 5 contributes to erythropoietin-mediated neuroprotection against hippocampal neuronal death after transient global cerebral ischemia. Neurobiol Dis. 2007. 25: 45-53
112. Zhang HB, Cheng SX, Tu Y, Zhang S, Hou SK, Yang Z. Protective effect of mild-induced hypothermia against moderate traumatic brain injury in rats involved in necroptotic and apoptotic pathways. Brain Inj. 2017. 31: 406-15