- Department of Neurosurgery, St Lukas Hospital in Tarnów, Poland,
- Department of Childrens’ Neurosurgery, Faculty of Medicine, Jagiellonian University Medical College, Kraków, Poland.
Correspondence Address:
Paula Piątek, Department of Neurosurgery, St Lukas Hospital in Tarnów, Poland.
DOI:10.25259/SNI_365_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Paula Piątek1, Stanisław Kwiatkowski2, Olga Milczarek2. Spinal meningiomas in pediatric patients – A case series and literature review. 30-Sep-2022;13:445
How to cite this URL: Paula Piątek1, Stanisław Kwiatkowski2, Olga Milczarek2. Spinal meningiomas in pediatric patients – A case series and literature review. 30-Sep-2022;13:445. Available from: https://surgicalneurologyint.com/surgicalint-articles/11905/
Abstract
Background: Meningiomas are the most frequent intracranial tumors in the adult population; however, they are rare in pediatric patients. In children, meningiomas often require further diagnosis of genetic comorbidities. As many as, 50% of young patients with meningiomas suffer from neurofibromatosis type 2 (NF2). Spinal meningiomas include only 10% of pediatric meningiomas.
Case Description: Between 2000 and 2017, three children were hospitalized in the Neurosurgery Department. The patients reported prolonged periods of increasing neurological symptoms. In each case, a total gross tumor resection was performed. Histopathology result in each patient was meningioma psammomatosum. Only one girl required adjuvant radiotherapy (RTH) due to recurrent tumors. Magnetic resonance imaging (MRI) showed spinal nerves schwannomas and bilateral vestibular schwannomas in two patients with NF2.
Conclusion: A slow tumor growth is characteristic of spinal meningiomas. Back pain is a frequent initial symptom of a slowly growing tumor mass. Subsequently, neurological deficits gradually increase. Patients require a long follow-up period and control MRI-scan. Children with diagnosed spinal meningioma should be strictly controlled because of the high risk of their developing other tumors associated with NF2. Surgical resection is the primary treatment modality of meningiomas. Adjuvant RTH should be recommended only for selected patients.
Keywords: Neurofibromatosis type 2, Pediatric meningiomas, Spinal meningiomas
INTRODUCTION
Meningiomas are the most frequent intracranial tumors in adults accounting for 30% of all intracranial neoplasms.[
MATERIALS AND METHODS
Between the years 2000 and 2017, three patients below 18 years of age with spinal meningiomas were hospitalized and operated in the Pediatric Neurosurgery Department. The study group consisted of two girls and one boy. Two patients were diagnosed with NF2. The diagnosis of NF2 was based on the Manchester Clinical Diagnostic Criteria. Gene mutation research was not a part of the protocol. Data were obtained retrospectively from medical histories. Consent was granted by each patient and patient’s parents. Literature review was based on the data collected from the National Center of Biotechnology Information.
CLINICAL PRESENTATION
Case report 1
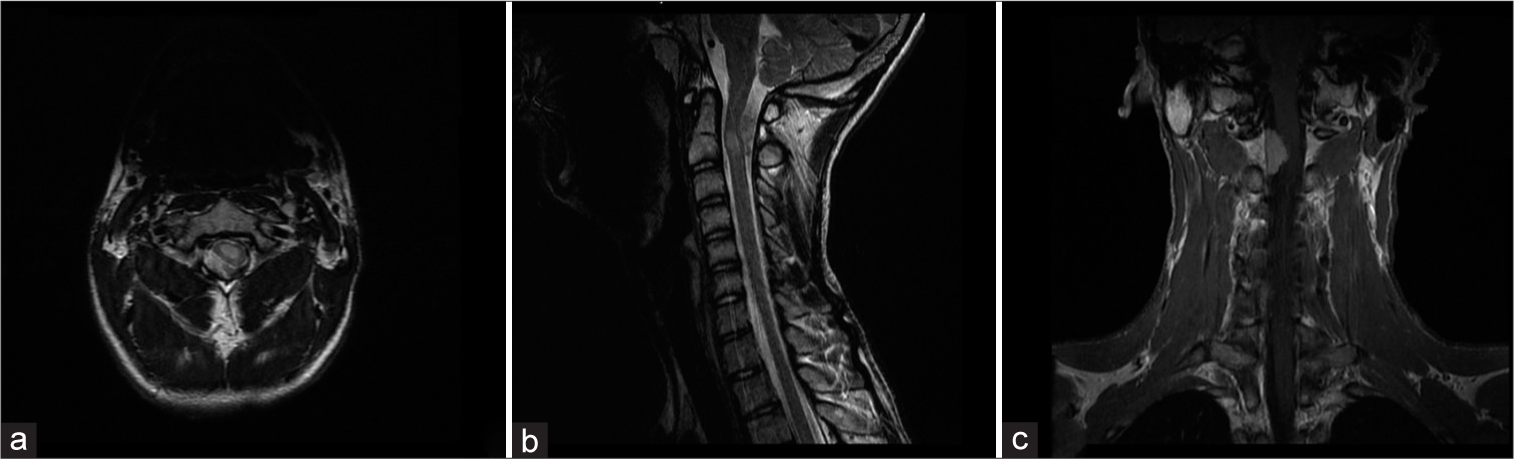
A 17-year-old boy was hospitalized for the diagnostic management of progressive focal neurological deficits. In clinical examination, he presented micro and retrognathia. In neurological examination, the patient presented right-side hemiparesis, impaired gait, and disturbances of superficial and deep sensation. When his medical history was taken, the patient reported past reconstruction of the temporomandibular joint. Magnetic resonance imaging (MRI) showed a tumor size 23 × 15 × 8 mm adhering to the posterior-lateral surface of the spinal cord at the C1/C2 level. The pathological mass showed strong enhancement after intravenous contrast medium administration. The tumor caused compression and displacement of the spinal cord. In the center of the spinal cord, the tumor was located at the C2 level [
Figure 1:
Preoperative magnetic resonance imaging (MRI) scans of Patient No. 1 with neurofibromatosis type 2: (a) Axial T2-weigthed MRI with contrast shows a meningioma 23 × 15 × 8 mm in size at the C1–2 level. The tumor adheres to the posterior-lateral side of the spinal canal. The meningioma presses and displaces the spinal cord. (b) Sagittal T2-weigthed MRI with contrast shows the meningioma and coexisting intraspinal ependymoma sized 11 × 9 × 5 mm at the C2 level. (c) Coronal T1-weigthed MRI with contrast shows the meningioma before surgery.
Case report 2
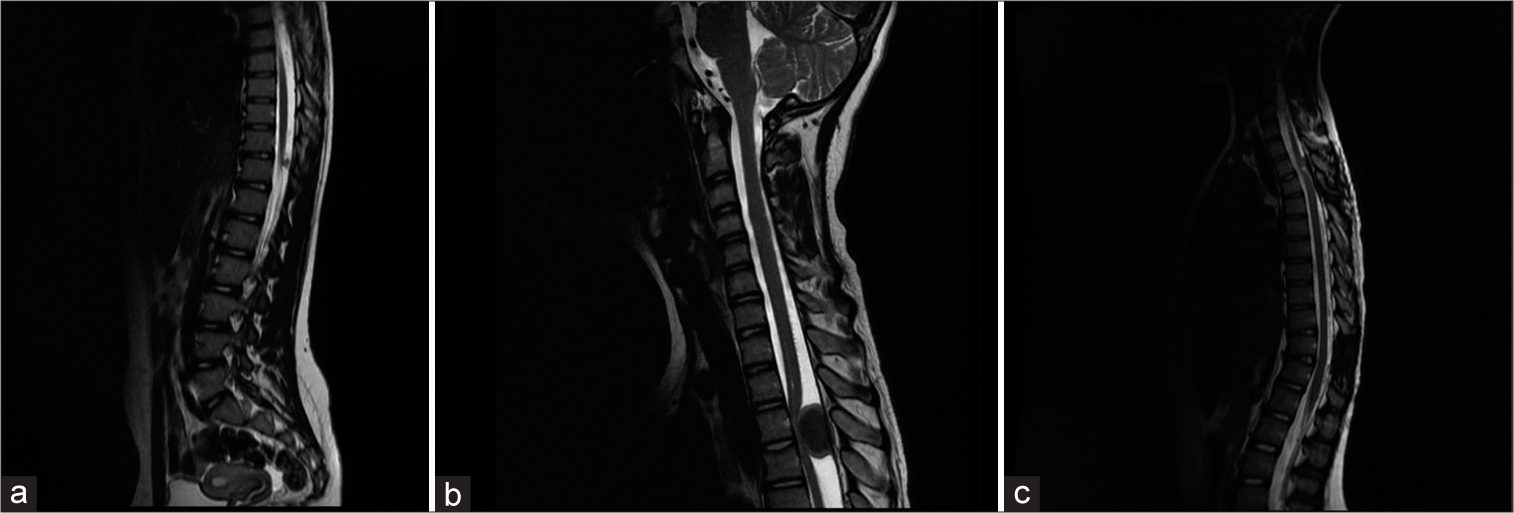
A 10-year-old girl with muscle weakness involving the lower limbs and unstable gait was hospitalized in the Neurosurgery Department. The neurological examination demonstrated spastic paraparesis of the lower limbs, bilateral plantar reflex, and lack of skin abdominal reflexes. The abnormalities presented on the left side included: clonus and foot drop, exaggerated ankle reflex, weakness of the knee reflex, and positive pronator drift. The patient presented normal superficial and deep sensation. The physical examination revealed muscular atrophy of the left calf. Thoracic MRI showed a small tumor 7 × 5 × 8 mm in size at the Th11 level [
Figure 2:
Pre- and post-operative imaging of patient No. 2: (a) Pre-operative sagittal T2-weigthed thoracic MRI of a 10-year-old girl. A small meningioma 7 × 5 × 8 mm at the Th11 level. (b) Sagittal T2-weigthed cervicothoracic junction MRI exposes a massive meningioma 24 × 11 × 15.5 mm at the Th3-Th4 level. The tumor presses and displaces the spinal cord. (c) Post-operative control sagittal T2-weigthed cervical and thoracic MRI. A post-operative reaction at the Th11 level. At the Th3/Th4 level, MRI shows a postoperative reaction or tumor residual mass. A MRI control scan was performed 6 months after the primary surgery.
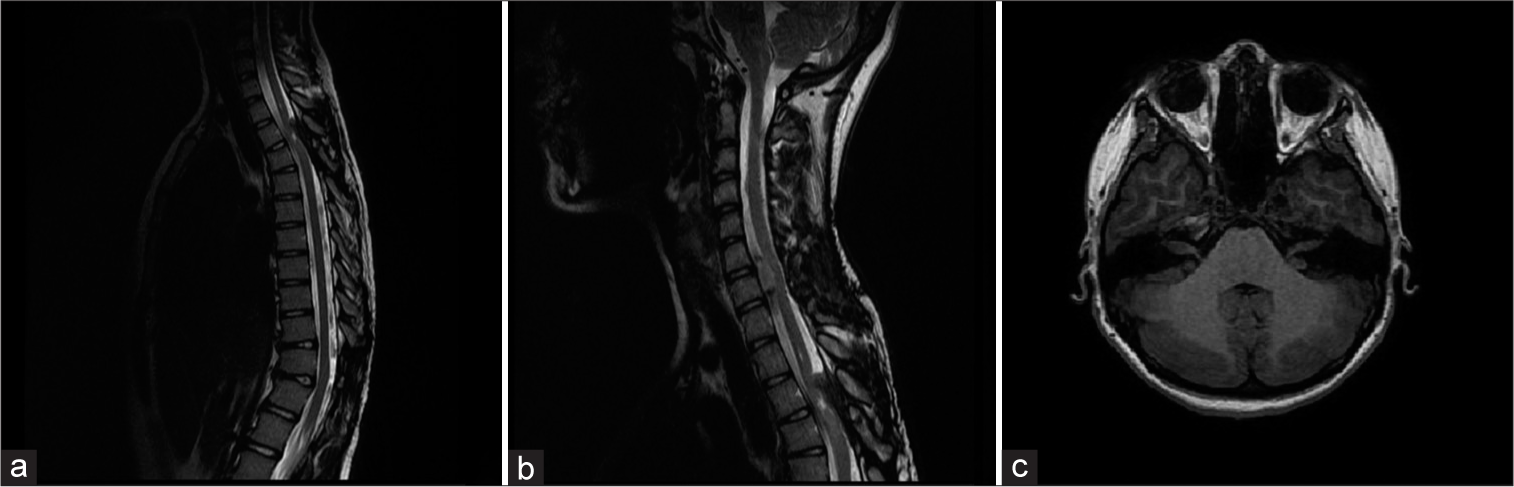
Figure 3:
MRI scan performed after 3-year follow-up of Patient No. 2: (a) Sagittal T2-weigthed thoracic MRI shows multiple small meningiomas at the Th2, Th5, Th8, Th9, Th11, Th12/L1 levels, and a tumor at the Th3-Th4 level sized 17 × 7 × 27 mm causing spinal cord compression. (b) Sagittal T2-weigthed cervical MRI presents tumors at the C2–3 and C6–7 levels with spinal cord compression. (c) Axial T2-weighed cervical MRI shows bilateral vestibular schwannomas.
Case report 3
A 14-year-old girl was admitted to hospital because of deterioration of the lower limbs paraparesis and back pain. The patient reported a 2-year history of symptom progression. A MRI scan showed a tumor at the Th4 level compressing the spinal cord. A laminectomy at the Th3-Th4 levels with tumor resection was performed. The histopathology result was meningioma psammomatosum. The symptoms gradually receded after the surgery. Three months after surgery, muscle strength of the lower limbs was rated as 4–5 points in the Lovett’s scale. In 2-year follow-up patients did not present further neurological deterioration. A control MRI scan did not show tumor recurrence or new tumors.
DISCUSSION
Pediatric meningiomas specification
Pediatric meningiomas are characterized by specific biological features including tumor histology, recurrence ratio, location, and prognosis as compared to tumors observed in the adult population.[
Histology
Pediatric meningiomas are a heterogeneous group of tumors. In children with NF2, meningiomas usually present atypical histological features, such as the papillary variant or clear cell type.[
High-grade tumors
At present, there is no effective treatment for pediatric patients with high-grade meningiomas. An alternative treatment of inaccessible tumors and histologically aggressive neoplasm is stereotactic radiosurgery (SRS). These tumors are rare and require special methods of treatment.[
Symptoms
Symptoms are mainly the result of spinal cord compression.[
Therapeutic strategy and treatment limitations
Patients with asymptomatic small benign meningiomas can be observed, but in symptomatic patients, complete surgical resection should be performed.[
Follow-up
Patients with NF2 are at a high risk of developing progressive and recurrent tumors that should be carefully monitored. Dirks et al. suggested a long postoperative follow-up period.[
CONCLUSION
Summarizing, spinal meningiomas are rare tumors in the pediatric population. In children, they often are the first manifestation of NF2. Therefore, the affected child should be under strict control and a MRI-scan should be periodically performed. Patients with asymptomatic small benign meningiomas can be observed, but in symptomatic patients, complete surgical resection should be performed. Adjuvant RTH in children should be recommended only for selected cases.
Author contributions
Paula Piątek: Substantial contribution to conception and design, acquisition of data, or analysis and interpretation of data; final approval of the version to be published; agreement to be accountable for all aspects of the work thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, drafting the article or revising it critically for important intellectual content.
Stanisław Kwiatkowski: substantial contribution to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content.
Olga Milczarek: senior author, substantial contribution to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Antinheimo J, Haapasalo H, Halite M, Tatagiba M, Thomas S, Brandis A. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J Neurosurg. 1997. 87: 610-4
2. Blakeley JO, Evans DG, Adler J, Brackmann D, Chen R, Ferner RE. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis Type 2. Am J Med Genet A. 2012. 158A: 24-41
3. 4. Chamberlain MC, Blumenthal DT. Intracranial meningiomas: Diagnosis and treatment. Expert Rev Neurother. 2004. 4: 641-8 5. Dirks MS, Butman JA, Kim HJ, Wu T, Morgan K, Tran AP. Long-term natural history of neurofibromatosis Type 2-associated intracranial tumours. J Neurosurg. 2012. 117: 109-17 6. Goutagny S, Kalamarides M. Meningiomas and neurofibromatosis. J Neurooncol. 2010. 99: 341-7 7. Grossbach AJ, Mahaney KB, Menezes AH. Pediatric meningiomas: 65-year experience at a single institution. J Neurosurg Pediatr. 2017. 20: 42-50 8. Horiba A, Hayashi M, Tamura N, Chiba K, Aihara Y, Kawamata T. Gamma knife treatment of malignant infantile brain tumors-case report. J Radiosurg SBRT. 2018. 5: 249-53 9. Kondziolka D, Madhok R, Lunsford LD, Mathieu D, Martin JJ, Niranjan A. Stereotactic radiosurgery for convexity meningiomas. J Neurosurg. 2009. 111: 458-63 10. Kotecha RS, Junckerstorff RC, Lee S, Cole CH, Gottardo NG. Pediatric 406 meningioma: Current approaches and future direction. J Neurooncol. 2011. 407: 1-10 11. Kotecha RS, Pascoe EM, Rushing EJ, Rorke-Adams LB, Zwerdling T, Gao X. Meningiomas in children and adolescents: A meta-analysis of individual patient data. Lancet Oncol. 2011. 12: 1229-39 12. Minniti G, Amichetti M, Enrici RM. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol. 2009. 4: 42 13. Norden AD, Drappatz J, Wen PY. Advances in meningioma therapy. Curr Neurol Neurosci Rep. 2009. 9: 231-40 14. Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur Spine J. 2008. 17: 1035-41 15. Santos MV, Furlanetti L, Valera ET, Brassesco MS, Tone LG, De Oliveira RS. Pediatric meningiomas: A single centre experience with 15 consecutive cases and review of literature. Chlid Nerv Syst. 2012. 28: 1887-96 16. Setzer M, Vatter H, Marquardt G, Seifert V, Vrionis FD. Management of spinal meningiomas: Surgical results and a review of the literature. Neurosurg Focus. 2007. 23: E14 17. Sheikh BY, Siqueira E, Dayel F. Meningioma in children: A report of nine cases and review of the literature. Surg Neurol. 1996. 45: 328-35 18. Thuijs NB, Uitdehaag BM, van Ouwerkerk WJ, van der Valk P, Vandertop WP, Peerdeman SM. Pediatric meningiomas in the Netherlands 1947-2010: A descriptive epidemiological case study. Childs Nerv Syst. 2012. 28: 1009-15 19. Traunecker H, Mallucci C, Grundy R, Pizer B, Saran F. Children’s Cancer and Leukaemia Group (CCLG): Guidelines for the management of intracranial meningioma in children and young people. Br J Neurosurg. 2008. 22: 13-25 discussion 24-5 20. Wang XQ, Zeng XW, Zhang BY, Dou YF, Wu JS, Jiang CC. Spinal meningioma in childhood: Clinical features and treatment. Child Nerv Syst. 2012. 28: 129-36 21. Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neurooncol. 2010. 99: 365-78 22. Wu L, Yang Ch, Liu T, Fang J, Yang J, Xu Y. Clinical features and long-term outcomes of pediatric spinal meningiomas. J Neurooncol. 2017. 133: 347-55