- Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, United States
- Global Neurosurgical Alliance, United States,
- College of Medicine, The University of Arizona College of Medicine - Tucson, Arizona, United States,
- Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States,
- Department of Neurosurgery, The University of Jordan School of Medicine, Amman, Jordan,
- Department of Medicine, Aga Khan University, Karachi, Pakistan,
- Department of Neurosurgery, University of Kufa College of Medicine, Kufa, Iraq,
- Department of Medicine, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom,
- College of Medicine, The University of Arizona College of Medicine - Phoenix, Arizona, United States.
Correspondence Address:
Albert Alan, Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States.
DOI:10.25259/SNI_776_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Barbara Buccilli1,2, Albert Alan2,3,4, Aljeradat Baha’2,5, Akmal Shahzad2,6, Yasser F. Almealawy2,7, Nathan Simbarashe Chisvo2,8, Michelle Ennabe2,9, Martin Weinand3,4. The importance of behavioral interventions in traumatic brain injury. 26-Jan-2024;15:22
How to cite this URL: Barbara Buccilli1,2, Albert Alan2,3,4, Aljeradat Baha’2,5, Akmal Shahzad2,6, Yasser F. Almealawy2,7, Nathan Simbarashe Chisvo2,8, Michelle Ennabe2,9, Martin Weinand3,4. The importance of behavioral interventions in traumatic brain injury. 26-Jan-2024;15:22. Available from: https://surgicalneurologyint.com/surgicalint-articles/the-importance-of-behavioral-interventions-in-traumatic-brain-injury/
Abstract
Background: Traumatic brain injury (TBI) poses a significant public health concern, profoundly impacting individuals and society. In this context, behavioral interventions have gained prominence as crucial elements in TBI management, addressing the diverse needs of TBI-affected individuals.
Methods: A comprehensive literature search was conducted, utilizing databases such as PubMed, Embase, and Scopus. Inclusion criteria encompassed studies focusing on behavioral interventions in TBI, with a particular emphasis on their impact on outcomes. Relevant articles published within the past decade were prioritized, and a qualitative synthesis of the findings was performed.
Results: Behavioral interventions have demonstrated their effectiveness in addressing various aspects of TBI care. They have been instrumental in improving cognitive functions, emotional stability, and adaptive behaviors among TBI patients. However, it is important to acknowledge that challenges still exist, including issues related to clinical heterogeneity and healthcare disparities.
Conclusion: The integration of behavioral interventions into standard clinical practice marks a transformative shift in TBI care. This approach holds immense potential for enhancing patient outcomes and elevating the overall quality of life for individuals grappling with the complexities of this condition. This review serves as a clarion call for healthcare practitioners, researchers, and policymakers to recognize the pivotal role of behavioral interventions in TBI care, advocating for their wider adoption to advance the field toward a more holistic and patient-centric approach.
Keywords: Behavior, Diet, Exercise, Neurorehabilitation, Nutrition
INTRODUCTION
Background and significance of traumatic brain injury (TBI)-related neuroprotection
TBI shows significant morbidity, disability, and mortality across all age groups.[
The initial damage triggers a local inflammatory response, primarily driven by microglia activation.[
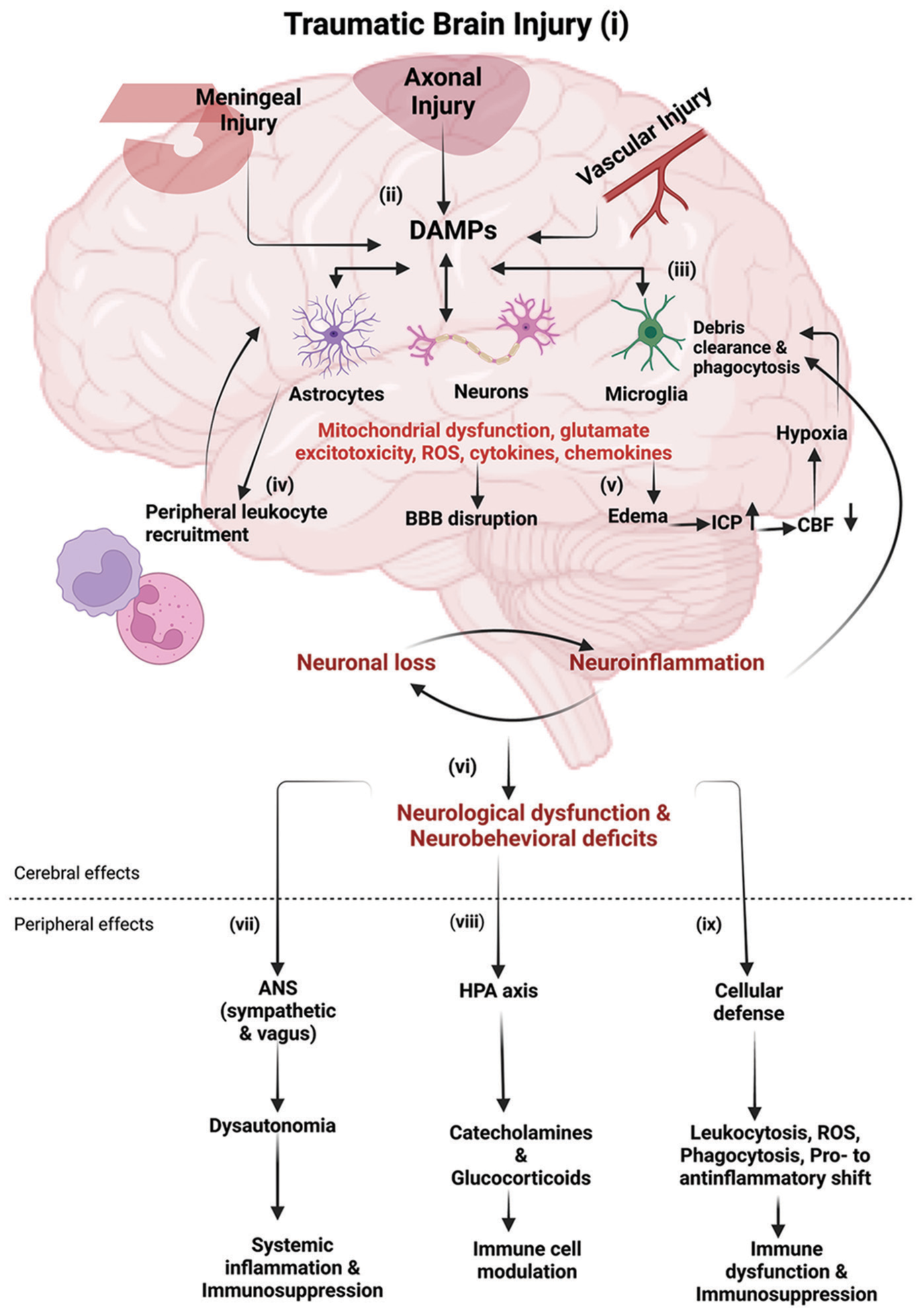
Figure 1:
Immune response following traumatic brain injury (TBI): (i-ii) following TBI, the primary mechanical injury can include meningeal contusion, axonal shearing, and cerebrovascular injury, culminating in meningeal and neuronal cell death, as well as microglial and astrocytic activation. (iii) Such neuronal injury and glial engagement generate chemokines, cytokines, and reactive oxygen species, along with the release of damage-associated molecular patterns (DAMPs), setting off an inflammatory response. (iv) In the presence of DAMPs, phagocytic microglia engage in debris clearance and synthesize neurotrophic agents. Sustained stimulation of these pathways induces subsequent injury through leukocyte recruitment, which initially aids in the removal of tissue debris. (v) Subsequently, it contributes to the progression of inflammation and disruption of the blood–brain barrier (BBB). The cytotoxic edema and compromised BBB integrity bring to an elevation of the intracranial pressure, leading to decreased cerebral blood flow, thereby intensifying hypoxia and disrupting the cerebral energy supply. Consequently, this cascade drives further neuronal depletion, propelling a self-perpetuating cycle of neuroinflammation and neurodegeneration. (vi) These progressive pathological modifications culminate in neurological dysfunction and deficits in motor, cognitive, and emotional functions. TBI also induces alterations in the autonomic nervous system (ANS), which monitors and regulates DAMPs, consequently eliciting both cerebral and peripheral immune responses. (vii) Activation of the sympathetic ANS culminates in the peripheral discharge of catecholamines (epinephrine and norepinephrine), which suppress the systemic immune responses of macrophages through the cholinergic anti-inflammatory pathway (CAO), thereby mitigating systemic inflammation. (viii) Furthermore, the release of catecholamines and glucocorticoids through the hypothalamic-pituitary-adrenal axis governs the functional behavior of systemic immune cells after TBI. (ix) The cellular immune response to traumatic brain injury involves an increase in leukocytosis and ROS generation, progresses through phagocytosis, and shifts from pro-inflammatory to anti-inflammatory states, potentially leading to immune dysfunction and immunosuppression. Abbreviations: ICP (increased intracranial pressure), CBF (cerebral blood flow), HPA (hypothalamic-pituitary-adrenal), ROS (reactive oxygen species). Image created with BioRender.com.
TBI’s social and financial costs have been drawing attention for years.[
Epidemiology and impact on the brain
TBI affects all ages, with a higher incidence in males.[
Mild TBI (mTBI) [
Table 1:
Classification of TBI severity based on the GCS score.[
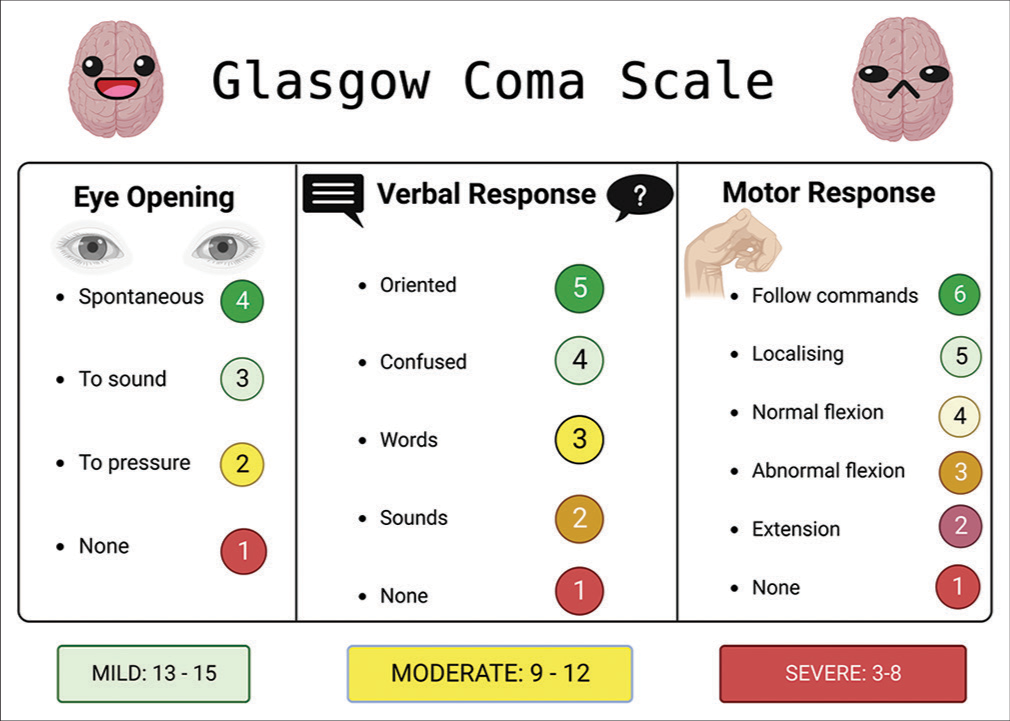
Figure 2:
This figure illustrates the Glasgow coma scale (GCS), a vital neurological assessment tool, as it pertains to traumatic brain injury (TBI). The GCS quantifies the patient’s level of consciousness based on eye, verbal, and motor responses, aiding clinicians in gauging TBI severity and guiding treatment decisions. Created with BioRender.com.
Scope and objectives of the review
The scope of this review is to comprehensively explore and analyze behavioral neuroprotection strategies that can be employed in the context of TBI and represent an important aid to surgical and pharmacological therapies. By investigating the available literature and clinical trials, the review seeks to provide a comprehensive overview of the strengths and limitations of each strategy and their potential for translation into clinical practice. Ultimately, the objectives of this review are to inform healthcare practitioners, researchers, and policymakers about evidence-based neuroprotection strategies that hold promise in alleviating the consequences of TBI and fostering better patient recovery and quality of life.
METHODOLOGY
Inclusion and exclusion criteria
The following inclusion criteria have been applied to ensure the relevance and comprehensiveness of the review. We included peer-reviewed research articles, clinical trials, systematic reviews, and meta-analyses published in the English language. The articles had to be focused on patients diagnosed with TBI and on behavioral interventions as a primary or adjunctive treatment strategy for TBI.
To maintain the rigor of the review, the following exclusion criteria have been applied. Non-peer-reviewed articles, conference abstracts, and editorial/opinion pieces were excluded, as well as studies lacking relevant data on the impact of behavioral interventions on TBI outcomes.
Search strategy
A comprehensive literature search will be conducted in multiple electronic databases, including PubMed/MEDLINE and Scopus. The search strategy will use a combination of Medical Subject Headings terms and keywords related to “traumatic brain injury” and “behavioral interventions.” The search strategy will be adapted for each database and reviewed by at least two members of the team for accuracy and completeness. Example search terms include: (“traumatic brain injury” OR “TBI” OR “head injury” OR “brain trauma”) AND (“behavioral intervention” OR “rehabilitation” OR “exercise” OR “sleep”).
Study selection and quality assessment
Two independent reviewers initially screened the titles and abstracts of all identified articles to determine their relevance based on the inclusion and exclusion criteria. Full-text articles have been retrieved for further evaluation if they meet the initial screening criteria. Any discrepancies between the reviewers regarding article inclusion will be resolved through discussion or consultation with a third reviewer if necessary.
The quality of each included study will be assessed using appropriate tools such as the Risk of Bias 2. The quality assessment will guide the interpretation of study findings.
Data analysis and reporting
Data synthesis will involve a narrative summary of the findings. The results of this review will be reported in a structured manner, including tables, figures, and a narrative synthesis.
Ethical considerations
As this review involves the analysis of existing published data, ethical approval is not required. However, ethical principles of data confidentiality and proper citation will be adhered to throughout the review process.
LIFESTYLE-BASED INTERVENTIONS
Recent research has provided us with invaluable insights into the intricate relationship between lifestyle factors and TBI, shedding light on the complex interplay between diet, exercise, lifestyle interventions, and post-TBI outcomes. This evolving body of research not only deepens our understanding of the challenges faced by individuals with TBI but also highlights the potential for targeted interventions that could significantly improve their quality of life.
Examination of lifestyle factors influencing TBI outcome
In one study, the incidence of preinjury stressful life events was a strong predictor of outcome, and a history of posttraumatic stress symptoms was associated with lower scores on the mental health component of the Short-Form Health Survey (SF-36). These findings highlight the importance of assessing preinjury stress and posttraumatic stress symptoms to identify those at risk for poor outcomes after mTBI.[
A preclinical study examined the effects of social and environmental enrichment on outcomes in a pediatric TBI model. It demonstrated that enhanced social and cognitive environments could lead to improved long-term outcomes. Environmental enrichment increased sensorimotor performance and sociosexual interactions, while social housing reduced hyperactivity and anxiety-like behaviors.[
Nutrition
In one study, French maritime pine bark extract supplementation significantly reduced Interleukin (IL)-6, IL-1b, and C-reactive protein levels in the intervention group of TBI patients compared to the control group. Clinical scores and the Nutric score were improved in the intervention group, leading to a 15% higher survival rate.[
The potential of ketogenic diets (KD) in acute neurotraumatic events was recently explored, and KD demonstrated benefits in improving motor neurorecovery, gray matter sparing, pain thresholds, and neuroinflammation, with effects likely linked to cellular energetics, mitochondria function, and inflammation modulation.[
On the other hand, preclinical evidence suggested benefits from adding substantial amounts of omega-3 fatty acids (n-3FAs) to improve outcomes in TBI patients.[
Preinjury supplementation with creatine has also been shown to boost the availability of energy, alleviate oxidative stress, and uphold the balance of energy within mitochondria; the potential beneficial impacts of creatine include its direct influence on maintaining mitochondrial energy levels and regulating neurotransmitter receptors. Another nutrient is curcumin, which is present in turmeric and recognized for its antioxidative and anti-inflammatory characteristics. Curcumin can shield the brain against lipid peroxidation and oxidative stress. Moreover, it counteracts inflammatory pathways by inhibiting the activation of nuclear factor-kappa B (NF-kB) and the release of proinflammatory cytokines.[
Oligonol, a phenolic product derived from polyphenols, was studied as a protective factor against oxidative stress, apoptosis, and neurodegenerative disorders.[
Researchers investigated the effects of branched-chain amino acid (BCAA) supplementation in severe TBI patients. BCAAs were found to enhance cognitive recovery and reduce cognitive decline without negative effects on precursor amino acids.[
In a clinical case report, short-term parenteral nutrition with fat emulsion was linked to the development of hemophagocytosis and multiple organ failure in a TBI patient. The authors suggested that fat retention or agglutination of fat particles might contribute to this outcome.[
A study examining the combined effects of progesterone and Vitamin D on recovery after TBI in middle-aged rats showed potential benefits in preserving spatial and reference memory, reducing neuronal loss, and preventing certain pathophysiological consequences of brain injury.[
Wogonin, a flavonoid with anti-inflammatory properties, was investigated for its effects on functional outcomes, brain edema, and inflammatory pathways following TBI. Wogonin treatment improved functional recovery, reduced brain edema, and attenuated the TLR4/NF-kB-mediated inflammatory response in mice.[
A different study investigated the effects of a low-protein and high-carbohydrate (LPHC) diet on a mouse model of Parkinson’s disease (PD) induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).[
Supplementation with nicotinamide adenine dinucleotide (NAD [+]) and its precursors as a strategy to prevent cognitive decline across various disease contexts was also explored. The review summarized the research findings for different sources of cognitive impairment, including age-related cognitive decline, Alzheimer’s disease, vascular dementia, diabetes, stroke, and TBI. The review mentioned that NAM administration in rat models demonstrated potential benefits, including reduced lesion sizes and diminished sensory, motor, and cognitive deficits. The study also highlighted positive outcomes observed in animal models. Still, it emphasized the need for controlled clinical research to determine the efficacy of NAD (+) precursor supplementation in addressing cognitive health in humans.[
In a randomized, double-blind, placebo-controlled, and crossover trial, the effects of brain-directed nutrients (BDNs) on cerebral blood flow (CBF) and neuropsychological testing were investigated.[
In a pilot trial, the effects of l-carnitine on biomarkers of injury in patients with TBI were investigated.[
Using a rat model of TBI, a study examined the potential neuroprotective role of pyrroloquinoline quinone (PQQ).[
Researchers aimed to explore the neuroprotective effects of phospholipid precursors administered postinjury in a study.[
Using a cold injury model in mice, a study investigated the effects of lutein/zeaxanthin isomers (L/Zi) isomers on brain injury outcomes.[
Researchers explored the effects of Hericium erinaceus and Coriolus versicolor in a mouse model of TBI. TBI was induced in mice using controlled cortical impact, resulting in decreased expression of tyrosine hydroxylase and dopamine transporter in the substantia nigra, accompanied by behavioral alterations. Daily oral treatment with H. erinaceus and C. versicolor restored behavioral deficits and prevented the decrease in tyrosine hydroxylase and dopamine transporter expression. Moreover, the vehicle groups showed increased neuroinflammation and oxidative stress, both of which were mitigated by the fungal treatments. This study suggested that TBI may trigger the potential neurodegenerative events associated with PD and that nutritional fungi such as H. erinaceus and C. versicolor could play a role in neuroprotection, attenuating neuroinflammation and oxidative stress processes.[
In a subsequent study, researchers investigated the neuroprotective effects of cinnamon polyphenol extract in a mouse model of TBI. Cinnamon polyphenol extract administration significantly reduced infarct and edema formation post-TBI. This reduction was associated with alterations in inflammatory and oxidative parameters, including NF-kB, IL 1-beta, IL 6, nuclear factor erythroid 2-related factor 2, and antioxidant enzymes. These results suggested that cinnamon polyphenol extract exerted neuroprotective effects by suppressing inflammation and oxidative injury, thus holding potential as a therapeutic agent for TBI.[
Furthermore, the focus shifted to the potential therapeutic intervention of exogenous ketones and lactate for brain injury and neurodegenerative conditions. These preparations showed promise as therapeutic adjuncts for both acute and chronic neurological conditions, with the ability to modulate brain function and potentially mitigate neurodegenerative risks.[
In a study using a mouse model of mTBI, the protective effects of n-3 PUFAs were examined. Fat-1 mice, which synthesize n-3 PUFA endogenously, showed significantly lower neurological severity scores and greater neurological restoration compared to wild-type mice. These findings suggested the potential protective role of n-3 PUFA against mild brain injury.[
Finally, the neuroprotective potential of icariin, a component of Epimedii Herba, was investigated in a mouse model of TBI. Icariin treatment led to improved sensory-motor and cognitive function in various tests. This effect was associated with the upregulation of synaptic plasticity markers, suggesting a possible mechanism for icariin’s effects on functional recovery after TBI.[
Similarly, the effects of folinic acid were studied in a rat model of head injury. Folinic acid administration reduced serum levels of homocysteine, tumor necrosis factor (TNF)-a, IL-10, and HMGB1 gene expression, indicating potential anti-inflammatory properties. This study suggested that folinic acid might mitigate neuroinflammation associated with TBI.[
Exercise
Studies have shown a neuroprotective effect of exercise in conditions such as stroke, with benefits including promotion of angiogenesis, inhibition of inflammatory response, and protection of the BBB.[
Aerobic exercise post-TBI can reduce neuronal injury, as has been shown in animal studies, enhance neuroprotective trophic factors, and improve neuronal survival. Subsymptom threshold exercise has also been found to be safe and effective in decreasing symptom burden in individuals with mTBI. However, the timing of exercise initiation is important, as early exercise in the acute postinjury period might hinder recovery mechanisms. Although limited human clinical studies exist, aerobic exercise post-TBI is believed to engage cerebrovascular mechanisms and provide neurophysiologic benefits to mitigate post-TBI changes. In addition, exercise counteracts the negative effects of prolonged inactivity and physical deconditioning.[
Previous exercise training was shown to alter oxidative-inflammatory status in the liver, protect against hepatic inflammation and oxidative stress, and improve mitochondrial function in a rat model of TBI. In the same model, exercise also had positive effects on cognitive signaling pathways and reduced levels of circulating and neuronal cytokines.[
Similarly, the neuroprotective effects of endurance exercise on neuroinflammation in a mouse model of PD were found to have neuroprotective effects against neuroinflammation in PD mice as exercise reduced a-synuclein protein levels, proinflammatory cytokines, and improved dopaminergic function.[
Furthermore, the relationship between physical activity, global health, and cognitive health was studied in individuals with a history of TBI. The study found that physical activity was associated with improved global and cognitive health perceptions, particularly in individuals with a history of TBI. The research highlighted the potential of physical activity programs to promote better health outcomes in TBI survivors.[
Subsequent research efforts explored the effects of voluntary wheel running on object recognition memory and neuroprotection after controlled cortical impact injury. Different exercise protocols were evaluated, and the results showed that exercise improved object recognition memory and reduced neurodegeneration.[
In the study titled “Characterizing Physical Activity and Sedentary Behavior in Adults with persistent postconcussive symptoms after mTBI,” researchers evaluated the physical activity and sedentary behavior of adults with persistent postconcussive symptoms after mTBI. Physical activity was decreased in individuals with persistent symptoms, and meeting physical activity guidelines was associated with better clinical outcomes and improved quality of life.[
Different research investigated the effects of posttraumatic exercise initiation on outcomes after moderate TBI using a mouse-controlled cortical impact model. The study compared late exercise initiation at five weeks posttrauma with early exercise initiation at one week. Late exercise significantly reduced memory impairment and lesion volume, along with attenuating classical inflammatory pathways, activating alternative inflammatory responses, and enhancing neurogenesis. In contrast, early exercise did not alter behavioral recovery or lesion size and even increased neurotoxic proinflammatory responses.[
On the other hand, in the study titled “Moderate Intensity Treadmill Exercise Increases Survival of Newborn Hippocampal Neurons and Improves Neurobehavioral Outcomes after TBI,” researchers used a mouse-controlled cortical impact model to assess the effects of treadmill exercise on functional outcome and hippocampal neural proliferation after brain injury. The study demonstrated that moderate-intensity treadmill exercise initiated after brain injury reduced anxiety-like behavior, improved spatial memory, and promoted hippocampal proliferation and newborn neuronal survival without altering pathophysiological measures such as lesion volume and axon degeneration.[
The role of voluntary physical exercise and citicoline after TBI in a rat model was that citicoline and exercise had separate neuroprotective effects, including improved memory deficits and increased neurogenesis. However, the effects of citicoline and exercise did not synergize and even interfered with each other in some measures.[
Sleep
Melatonin, a sleep-regulating and neuroprotective agent, has been explored as a potential treatment for TBI-induced sleep dysfunction due to its anti-inflammatory properties and ability to modulate circadian rhythms. However, the lack of standardization in melatonin research has posed challenges in translating findings into effective treatments for TBI-related sleep issues.[
Comparing healthy controls and individuals with mTBI, it was observed that control participants had higher physical activity and lower sleep time compared to the mTBI group.[
Sleep deprivation after a TBI can have a neuroprotective role, leading to reduced morphological damage and enhanced recovery in rats. This counterintuitive finding suggests that wakefulness during the recovery process may promote neuroprotection.[
Neurorehabilitation strategies to promote neuroprotection and recovery
The combined intervention of manualized cognitive rehabilitation (compensatory cognitive training) and supported employment have been shown to significantly improve return-to-work (RTW) rates, reduce time to RTW, enhance work stability, and improve work productivity. In addition, improvements were observed in self-reported symptoms, emotional and cognitive function, and quality of life of patients with mild to moderate TBI and postconcussive symptoms.[
The effectiveness of executive functions (EF) training for adults with TBI within a virtual supermarket was examined. The study focused on using a virtual reality (VR) supermarket for EF training compared to conventional occupational therapy. Both groups showed improvements, but the VR group exhibited greater improvement in complex everyday activities. The study highlighted the potential of VR-based interventions for cognitive rehabilitation after TBI.[
On the other hand, a randomized controlled trial aimed to assess the efficacy of a 12-week health and wellness group intervention for individuals with moderate to severe TBI. The intervention’s impact on health-promoting lifestyle changes was evaluated. However, the study results indicated no significant differences between treatment and control groups in terms of health and wellness outcomes. Factors such as individualized health goals and outcome measures might have influenced the intervention’s effectiveness.[
In a community-based healthy lifestyle intervention study, individuals with TBI were examined. The program focused on achieving weight loss through increased physical activity and improved dietary behaviors. Participants showed high adherence to the program, resulting in significant weight loss and improvements in physiologic outcomes. However, self-reported health, quality of life, and step count did not show significant changes. The study highlighted the potential success of healthy lifestyle interventions for individuals with TBI.[
Plasma amino acid levels in severe TBI patients after rehabilitation, it was found that levels of plasma tyrosine and several essential amino acids remained lower than normal even after two months of rehabilitation. The study suggested that these amino acid abnormalities persisted despite the rehabilitation period.[
Finally, a case study explored the behavioral treatment of pulsatile tinnitus and headache after traumatic head injury. The evaluation included a polygraphic assessment of vasomotor and electromyographic function before and after treatment. Results showed that a combination of lifestyle modifications and specific behavioral interventions successfully improved self-report indices of functioning and the underlying physiology related to the disorder. The study highlighted the potential value of including polygraphic assessment in the treatment and evaluation of pulsatile tinnitus.[
A multidisciplinary approach is also important in TBI management. The addition of a dedicated physiatrist specialized in brain injury medicine and functional outcomes following TBI was associated with improved functional outcomes on discharge from rehabilitation. Furthermore, the presence of a dedicated physiatrist led to changes in neuroprotective medication management in the acute care setting.[
CONCLUSION
This comprehensive review has delved into the multifaceted landscape of behavioral interventions in the management of TBI. The overarching aim was to elucidate the significance of these interventions and their impact on patient outcomes.
The literature explored herein underscores the paramount importance of behavioral interventions as a crucial component of TBI rehabilitation. TBI, with its diverse clinical manifestations and long-term consequences, necessitates a holistic approach to care. Behavioral interventions can yield substantial benefits across various domains. Cognitive rehabilitation programs have shown promise in ameliorating cognitive deficits, enhancing EF, and improving overall cognitive performance. Psychotherapeutic approaches, such as cognitive-behavioral therapy, have proven effective in addressing emotional and psychological sequelae, including depression, anxiety, and posttraumatic stress disorder. Furthermore, behavior modification strategies have contributed to the management of behavioral issues and the facilitation of adaptive behaviors.
It is noteworthy that the effectiveness of these interventions often depends on several factors, including the timing of intervention, patient characteristics, and the integration of multiple modalities. Individualized care tailored to the unique needs of each TBI patient is paramount.
However, as with any therapeutic approach, challenges persist. The heterogeneity of TBI presentations and the need for personalized interventions pose clinical complexities. In addition, logistical barriers, including access to specialized care and healthcare disparities, warrant attention. Despite these challenges, the potential for improving the lives of TBI patients through behavioral interventions remains substantial. This review underscores the need for continued research, innovation, and the development of standardized protocols to optimize the delivery of these interventions.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Adkins DL, Ferguson L, Lance S, Pevtsov A, McDonough K, Stamschror J. Combining multiple types of motor rehabilitation enhances skilled forelimb use following experimental traumatic brain injury in rats. Neurorehabil Neural Repair. 2015. 29: 989-1000
2. Amen DG, Taylor DV, Ojala K, Kaur J, Willeumier K. Effects of brain-directed nutrients on cerebral blood flow and neuropsychological testing: A randomized, double-blind, placebo-controlled, crossover trial. Adv Mind Body Med. 2013. 27: 24-33
3. Amorós-Aguilar L, Portell-Cortés I, Costa-Miserachs D, Torras-Garcia M, Riubugent-Camps E, Almolda B. The benefits of voluntary physical exercise after traumatic brain injury on rat’s object recognition memory: A comparison of different temporal schedules. Exp Neurol. 2020. 326: 113178
4. Aquilani R, Iadarola P, Boschi F, Pistarini C, Arcidiaco P, Contardi A. Reduced plasma levels of tyrosine, precursor of brain catecholamines, and of essential amino acids in patients with severe traumatic brain injury after rehabilitation. Arch Phys Med Rehabil. 2003. 84: 1258-65
5. Aquilani R, Iadarola P, Contardi A, Boselli M, Verri M, Pastoris O. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005. 86: 1729-35
6. Aruoma OI, Sun B, Fujii H, Neergheen VS, Bahorun T, Kang KS. Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors. 2006. 27: 245-65
7. Bell A, Hewins B, Bishop C, Fortin A, Wang J, Creamer JL. Traumatic brain injury, sleep, and melatonin-intrinsic changes with therapeutic potential. Clocks Sleep. 2023. 5: 177-203
8. Black EK, Phillips JK, Seminetta J, Bailes J, Lee JM, Finan JD. The effect of dietary supplementation with high-or low-dose omega-3 fatty acid on inflammatory pathology after traumatic brain injury in rats. Transl Neurosci. 2021. 12: 76-82
9. Bonsale R, Infantino R, Perrone M, Marabese I, Ricciardi F, Fusco A. The long-term exercise after traumatic brain injury: Reharmonizing brain by sound body. Brain Res. 2023. 1816: 148471
10. Brenner LA, Braden CA, Bates M, Chase T, Hancock C, Harrison-Felix C. A health and wellness intervention for those with moderate to severe traumatic brain injury: A randomized controlled trial. J Head Trauma Rehabil. 2012. 27: E57-68
11. Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: Effects of moderate hypothermia. J Neurotrauma. 2007. 24: 1707-17
12. Campbell JM. Supplementation with NAD(+) and its precursors to prevent cognitive decline across disease contexts. Nutrients. 2022. 14: 3231
13. Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004. 36: 28-60
14. Cheatwood JL, Burnet D, Butteiger DN, Banz WJ. Soy protein diet increases skilled forelimb reaching function after stroke in rats. Behav Brain Res. 2011. 216: 681-4
15. Chen CC, Hung TH, Wang YH, Lin CW, Wang PY, Lee CY. Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kB signaling after experimental traumatic brain injury. PLoS One. 2012. 7: e30294
16. Chen J, Zhu T, Yu D, Yan B, Zhang Y, Jin J. Moderate intensity of treadmill exercise rescues TBI-induced ferroptosis, neurodegeneration, and cognitive impairments via suppressing STING pathway. Mol Neurobiol. 2023. 60: 4872-96
17. Chio CC, Lin HJ, Tian YF, Chen YC, Lin MT, Lin CH. Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-kB/IL-6/synapsin I axis in traumatic brain injury rats. J Neuroinflammation. 2017. 14: 90
18. Chou W, Liu YF, Lin CH, Lin MT, Chen CC, Liu WP. Exercise rehabilitation attenuates cognitive deficits in rats with traumatic brain injury by stimulating the cerebral HSP20/BDNF/TrkB signalling axis. Mol Neurobiol. 2018. 55: 8602-11
19. Chu C, Li T, Yu L, Li Y, Li M, Guo M. A low-protein, high-carbohydrate diet exerts a neuroprotective effect on mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson’s disease by regulating the microbiota-metabolite–brain axis and fibroblast growth factor 21. J Agric Food Chem. 2023. 71: 8877-93
20. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015. 72: 355
21. D’Amico R, Salinaro AT, Fusco R, Cordaro M, Impellizzeri D, Scuto M. Hericium erinaceus and Coriolus versicolor modulate molecular and biochemical changes after traumatic brain injury. Antioxidants. 2021. 10: 898
22. De Castro MRT, Ferreira AP, Busanello GL, da Silva LR, da Silveira Junior ME, Fiorin FD. Previous physical exercise alters the hepatic profile of oxidative-inflammatory status and limits the secondary brain damage induced by severe traumatic brain injury in rats. J Physiol. 2017. 595: 6023-44
23. Desai A, Park T, Barnes J, Kevala K, Chen H, Kim HY. Reduced acute neuroinflammation and improved functional recovery after traumatic brain injury by a-linolenic acid supplementation in mice. J Neuroinflammation. 2016. 13: 253
24. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019. 130: 1080-97
25. Dill LK, Teymornejad S, Sharma R, Bozkurt S, Christensen J, Chu E. Modulating chronic outcomes after pediatric traumatic brain injury: Distinct effects of social and environmental enrichment. Exp Neurol. 2023. 364: 114407
26. Driver S, Reynolds M, Woolsey A, Callender L, Prajapati PK, Bennett M. Impact of a community-based healthy lifestyle program on individuals with traumatic brain injury. J Head Trauma Rehabil. 2018. 33: E49-58
27. Faul M, Coronado V. Epidemiology of traumatic brain injury. Handb Clin Neurol. 2015. 127: 3-13
28. Faul M, Wald MM, Xu L, Coronado VG. Traumatic brain injury in the United States; Emergency department visits, hospitalizations, and deaths, 2002-2006. 2010. p.
29. Feigin VL, Theadom A, Barker-Collo S, Starkey NJ, McPherson K, Kahan M. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013. 12: 53-64
30. Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010. 27: 497-507
31. Fukui K. Neuroprotective and anti-obesity effects of tocotrienols. J Nutr Sci Vitaminol (Tokyo). 2019. 65: S185-7
32. Gan TT, Liao Q, Wang JH, Fan ZH, Cao J, Pan HJ. Neuroprotective effects of voluntary exercise and Yisaipu after traumatic brain injury in mice. Sheng Li Xue Bao. 2022. 74: 333-52
33. Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012. 4: 134ra60
34. Goodman JC, Van M, Gopinath SP, Robertson CS. Proinflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl. 2008. 102: 437-9
35. Greiss C, Yonclas PP, Jasey N, Lequerica A, Ward I, Chiaravalloti N. Presence of a dedicated trauma center physiatrist improves functional outcomes following traumatic brain injury. J Trauma Acute Care Surg. 2016. 80: 70-5
36. Griesbach GS. Exercise after traumatic brain injury: Is it a double-edged sword?. PM R. 2011. 3: S64-72
37. Gunal MY, Sakul AA, Caglayan AB, Erten F, Kursun OE, Kilic E. Protective effect of lutein/zeaxanthin isomers in traumatic brain injury in mice. Neurotox Res. 2021. 39: 1543-50
38. Hanscom M, Loane DJ, Shea-Donohue T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest. 2021. 131: e143777
39. Hegel MT, Martin JB. Behavioral treatment of pulsatile tinnitus and headache following traumatic head injury. Behav Modif. 1998. 22: 563-72
40. Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020. 40: 2960-74
41. Hicks RR, Boggs A, Leider D, Kraemer P, Brown R, Scheff SW. Effects of exercise following lateral fluid percussion brain injury in rats. Restor Neurol Neurosci. 1998. 12: 41-7
42. Howe EI, Langlo KP, Terjesen HC, Røe C, Schanke AK, Søberg HL. Combined cognitive and vocational interventions after mild to moderate traumatic brain injury: Study protocol for a randomized controlled trial. Trials. 2017. 18: 483
43. Iankova A. The Glasgow coma scale clinical application in emergency departments. Emerg Nurse. 2006. 14: 30-5
44. Ibeh S, Bakkar NM, Ahmad F, Nwaiwu J, Barsa C, Mekhjian S. High fat diet exacerbates long-term metabolic, neuropathological, and behavioral derangements in an experimental mouse model of traumatic brain injury. Life Sci. 2023. 314: 121316
45. Iyer KK, Zalesky A, Cocchi L, Barlow KM. Neural correlates of sleep recovery following melatonin treatment for pediatric concussion: A randomized controlled trial. J Neurotrauma. 2020. 37: 2647-55
46. Jacoby M, Averbuch S, Sacher Y, Katz N, Weiss PL, Kizony R. Effectiveness of executive functions training within a virtual supermarket for adults with traumatic brain injury: A pilot study. IEEE Trans Neural Syst Rehabil Eng. 2013. 21: 182-90
47. Jacotte-Simancas A, Costa-Miserachs D, Coll-Andreu M, Torras-Garcia M, Borlongan CV, Portell-Cortés I. Effects of voluntary physical exercise, citicoline, and combined treatment on object recognition memory, neurogenesis, and neuroprotection after traumatic brain injury in rats. J Neurotrauma. 2015. 32: 739-51
48. James SL, Theadom A, Ellenbogen RG, Bannick MS, MontjoyVenning W, Lucchesi LR. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. 18: 56-87
49. Jang Y, Koo JH, Kwon I, Kang EB, Um HS, Soya H. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017. 1655: 186-93
50. Johnson EM, Traver KL, Hoffman SW, Harrison CR, Herman JP. Environmental enrichment protects against functional deficits caused by traumatic brain injury. Front Behav Neurosci. 2013. 7: 44
51. Joo H, Bae J, Lee JS, Bang Y, Lee BJ, Park JW. Icariin improves functional behavior in a mouse model of traumatic brain injury and promotes synaptic plasticity markers. Planta Med. 2019. 85: 231-8
52. Karelina K, Schneiderman K, Shah S, Fitzgerald J, Velazquez Cruz R, Oliverio R. Moderate intensity treadmill exercise increases survival of newborn hippocampal neurons and improves neurobehavioral outcomes after traumatic brain injury. J Neurotrauma. 2021. 38: 1858-69
53. Keating CE, Cullen DK. Mechanosensation in traumatic brain injury. Neurobiol Dis. 2021. 148: 105210
54. Kim CC, Nakamura MC, Hsieh CL. Brain trauma elicits non-canonical macrophage activation states. J Neuroinflammation. 2016. 13: 117
55. Kossmann T, Stahel PF, Lenzlinger PM, Redl H, Dubs RW, Trentz O. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J Cereb Blood Flow Metab. 1997. 17: 280-9
56. Lecques JD, Kerr BJ, Hillyer LM, Kang JX, Robinson LE, Ma DW. N-3 polyunsaturated fatty acids ameliorate neurobehavioral outcomes post-mild traumatic brain injury in the fat-1 mouse model. Nutrients. 2021. 13: 4092
57. Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J. Incidence of traumatic brain injury across the full disease spectrum. Epidemiology. 2011. 22: 836-44
58. Lewis FD, Horn GJ. Traumatic brain injury: Analysis of functional deficits and posthospital rehabilitation outcomes. J Spec Oper Med. 2013. 13: 56-61
59. Lewis MD, Bailes J. Neuroprotection for the warrior: Dietary supplementation with omega-3 fatty acids. Mil Med. 2011. 176: 1120-7
60. Lewis MD. Concussions, traumatic brain injury, and the innovative use of omega-3s. J Am Coll Nutr. 2016. 35: 469-75
61. Lindsey GL, Yesen A, Christie AD. The impact of physical activity and sleep on physiology following a mTBI. Int J Exerc Sci. 2019. 12: 919-31
62. Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010. 31: 596-604
63. Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of posttraumatic hypothermia. J Neurotrauma. 2009. 26: 1123-34
64. Mahmoodpoor A, Shokouhi G, Hamishehkar H, Soleimanpour H, Sanaie S, Porhomayon J. A pilot trial of l-carnitine in patients with traumatic brain injury: Effects on biomarkers of injury. J Crit Care. 2018. 45: 128-32
65. Malekahmadi M, Shadnoush M, Islam SM, Shirvani A, Pahlavani N, Navashenaq JG. The effect of French maritime pine bark extract supplementation on inflammation, nutritional and clinical status in critically ill patients with traumatic brain injury: A randomized controlled trial. Phytother Res. 2021. 35: 5178-88
66. Martinez-Vargas M, Estrada Rojo F, Tabla-Ramon E, NavarroArgüelles H, Ortiz-Lailzon N, Hernández-Chávez A. Sleep deprivation has a neuroprotective role in a traumatic brain injury of the rat. Neurosci Lett. 2012. 529: 118-22
67. McDougall A, Bayley M, Munce SE. The ketogenic diet as a treatment for traumatic brain injury: A scoping review. Brain Inj. 2018. 32: 416-22
68. McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009. 68: 709-35
69. McKee AC, Stein TD, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013. 136: 43-64
70. Mercier LJ, Kowalski K, Fung TS, Joyce JM, Yeates KO, Debert CT. Characterizing physical activity and sedentary behavior in adults with persistent postconcussive symptoms after mild traumatic brain injury. Arch Phys Med Rehabil. 2021. 102: 1918-25.e1
71. Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr Opin Crit Care. 2002. 8: 101-5
72. Morris TP, Tormos Muñoz JM, Cattaneo G, Solana-Sánchez J, Bartrés-Faz D, Pascual-Leone A. Traumatic brain injury modifies the relationship between physical activity and global and cognitive health: Results from the barcelona brain health initiative. Front Behav Neurosci. 2019. 13: 135
73. Ng SY, Lee AY. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019. 13: 528
74. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD. The international incidence of traumatic brain injury: A systematic review and meta-analysis. Can J Neurol Sci. 2016. 43: 774-85
75. Oliver JM, Anzalone AJ, Turner SM. Protection before impact: The potential neuroprotective role of nutritional supplementation in sports-related head trauma. Sports Med. 2018. 48: 39-52
76. Omori NE, Woo GH, Mansor LS. Exogenous ketones and lactate as a potential therapeutic intervention for brain injury and neurodegenerative conditions. Front Hum Neurosci. 2022. 16: 846183
77. Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015. 157: 1683-96
78. Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis. 2013. 54: 252-63
79. Rafie F, Khaksari M, Amiresmaili S, Soltani Z, Pourranjbar M, Shirazpour S. Protective effects of early exercise on neuroinflammation, and neurotoxicity associated by traumatic brain injury: A behavioral and neurochemical approach. Int J Neurosci. 2022. 24: 1-14
80. Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002. 17: 1137-52
81. Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology. 2007. 69: 536-45
82. Roth B, Grände PO, Nilsson-Ehle P, Eliasson I. Possible role of short-term parenteral nutrition with fat emulsions for development of haemophagocytosis with multiple organ failure in a patient with traumatic brain injury. Intensive Care Med. 1993. 19: 111-4
83. Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008. 25: 719-38
84. Scheenen ME, van der Horn HJ, de Koning ME, van der Naalt J, Spikman JM. Stability of coping and the role of self-efficacy in the first year following mild traumatic brain injury. Soc Sci Med. 2017. 181: 184-90
85. Schmidt OI, Infanger M, Heyde CE, Ertel W, Stahel PF. The role of neuroinflammation in traumatic brain injury. Eur J Trauma. 2004. 30: 135-49
86. Sharma A, Muresanu DF, Ozkizilcik A, Tian ZR, Lafuente JV, Manzhulo I. Sleep deprivation exacerbates concussive head injury induced brain pathology: Neuroprotective effects of nano-wired delivery of cerebrolysin with a-melanocyte-stimulating hormone. Prog Brain Res. 2019. 245: 1-55
87. Sherzai AZ, Sherzai AN, Sherzai D. A systematic review of omega-3 consumption and neuroprotective cognitive outcomes. Am J Lifestyle Med. 2023. 17: 560-88
88. Shin MK, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K, Cintrón-Pérez CJ. Reducing acetylated tau is neuroprotective in brain injury. Cell. 2021. 184: 2715-32.e23
89. Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: A cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010. 113: 556-63
90. Tang H, Hua F, Wang J, Sayeed I, Wang X, Chen Z. Progesterone and vitamin D: Improvement after traumatic brain injury in middle-aged rats. Horm Behav. 2013. 64: 527-38
91. Taylor JM, Montgomery MH, Gregory EJ, Berman NE. Exercise preconditioning improves traumatic brain injury outcomes. Brain Res. 2015. 1622: 414-29
92. Te Ao B, Brown P, Tobias M, Ameratunga S, Barker-Collo S, Theadom A. Cost of traumatic brain injury in New Zealand: Evidence from a population-based study. Neurology. 2014. 83: 1645-52
93. Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 1976. 34: 45-55
94. Thau-Zuchman O, Gomes RN, Dyall SC, Davies M, Priestley JV, Groenendijk M. Brain phospholipid precursors administered post-injury reduce tissue damage and improve neurological outcome in experimental traumatic brain injury. J Neurotrauma. 2019. 36: 25-42
95. Tommy T, Islam AA, Hatta M, Bukhari A, Nasrullah , Adhimarta W. Effect of folinic acid on serum homocysteine, TNFa, IL-10, and HMGB1 gene expression in head injury model. Ann Med Surg (Lond). 2021. 65: 102273
96. Van Veldhoven LM, Sander AM, Struchen MA, Sherer M, Clark AN, Hudnall GE. Predictive ability of preinjury stressful life events and posttraumatic stress symptoms for outcomes following mild traumatic brain injury: Analysis in a prospective emergency room sample. J Neurol Neurosurg Psychiatry. 2011. 82: 782-7
97. White BA, Ivey JT, Velazquez-Cruz R, Oliverio R, Whitehead B, Pinti M. Exercise intensity and sex alter neurometabolic, transcriptional, and functional recovery following traumatic brain injury. Exp Neurol. 2023. 368: 114483
98. Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017. 16: 813-25
99. Yarar-Fisher C, Li J, Womack ED, Alharbi A, Seira O, Kolehmainen KL. Ketogenic regimens for acute neurotraumatic events. Curr Opin Biotechnol. 2021. 70: 68-74
100. Yulug B, Kilic E, Altunay S, Ersavas C, Orhan C, Dalay A. Cinnamon polyphenol extract exerts neuroprotective activity in traumatic brain injury in male mice. CNS Neurol Disord Drug Targets. 2018. 17: 439-47
101. Zafonte RD, Shih SL, Iaccarino MA, Tan CO. Neurologic benefits of sports and exercise. Handb Clin Neurol. 2018. 158: 463-71
102. Zhang P, Xianglei J, Hongbo Y, Zhang J, Xu C. Neuroprotection of early locomotor exercise poststroke: Evidence from animal studies. Can J Neurol Sci. 2015. 42: 213-20
103. Zhang P, Ye Y, Qian Y, Yin B, Zhao J, Zhu S. The effect of pyrroloquinoline quinone on apoptosis and autopha gy in traumatic brain injury. CNS Neurol Disord Drug Targets. 2017. 16: 724-36
104. Zhao Z, Sabirzhanov B, Wu J, Faden AI, Stoica BA. Voluntary exercise preconditioning activates multiple antiapoptotic mechanisms and improves neurological recovery after experimental traumatic brain injury. J Neurotrauma. 2015. 32: 1347-60